In such cases we would ask you to sign a Visual Elements licence agreement, tailored to the specific use you propose. Sulfur History Known to the ancients; referred to in Genesis as brimstone. Well they all begin with the letter S, and so does this week's element. As with sulfur oxidation, microbes can also gain energy from ferrous iron (Fe2+) or manganous manganese (Mn2+) oxidation. In addition of peptide bond Disulfide bond is a different type of covalent bond, is present in protein molecule. Please enable JavaScript to access the full features of the site. (Reprinted with permission from RICKARD, D. & LUTHER (III), G. W. 2007. A measure of how difficult it is to deform a material. WebWhat Is Disulfide Bond: Formation, Types, Functions. write an equation to show the interconversion between thiols and disulfides. Webwhat is the oxidation state of sulfur in a disulfidenate kaeding restaurant iowa city. 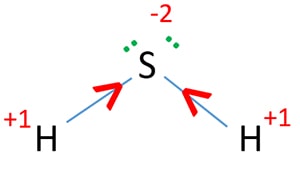 19). A spectrum of sulfur oxyanions with formal oxidation numbers ranging between polysulfide, S(-1), and sulfate, S(VI), (Table 2) occur in sediments. Sulfur oxidation (see Section 16.4.3.1) can be measured by addition of elemental sulfur (S0) to a sample and measuring the amount of sulfate (SO42) produced during incubation. A disulfide is a compound containing an -S-S- linkage. (A) Sox pathway, (B) branched thiosulfate oxidation pathway. lacked genes aprBA encoding APS reductase and sulfite oxidase that directly oxidizes sulfite to sulfate (Rohwerder etal., 2003). It is defined as being the charge that an atom would have if all bonds were ionic. Legal. These values were determined using several different methods. The percentage of a commodity which is recycled. Chemolithoautotrophic iron-oxidizing bacteria include the acidophilic aerobe Thiobacillus ferrooxidans. 6. Iron rapidly oxidizes to ferric (Fe3+) iron at neutral pH, so successful iron-oxidizers live in low pH environments, such as acid mine drainage, acid springs, mine tailings, or acid soils containing sulfide minerals such as pyrite. In the biochemistry lab, proteins are often maintained in their reduced (free thiol) state by incubation in buffer containing an excess concentration of b-mercaptoethanol (BME) or dithiothreitol (DTT). My son wouldn't eat eggs for 6 months when he got a smell of his first rotten one. The story of its discovery started when Rayleigh found that the nitrogen extracted from the air had a higher density than that made by decomposing ammonia. Sulfur and sulfate are non-toxic. However, all Acidithiobacillus spp. All in all, in both reactions we have a reasonably good nucleophile, an electron-poor, unhindered electrophile, and an excellent leaving group. The biogenic compound dimethylsulfide (DMS) is produced from the cleavage of dimethylsufonoprioponate, an osmotic regulatory compound produced by plankton in the ocean. WebSulfur oxides are the product of burning sulfur and oxygen. For more complex substance alkylthio is used instead of alkoxy. In fact, you wouldn't even double-count; Websulfur, organic and nitrogen compounds, periodicity, polymerization, rates of reaction, reaction kinetics, redox reactions and electrolysis, states of matter, transition elements worksheets for college and university revision redox, and oxidation. [(oxidation number of sulfur) X 1] + [(+1) X 2] + [(-2) X 4] = 0. thiooxidans, which was the first acidophile to be described in 1919. He was left with one percent which would not react and found it was denser than nitrogen. Interestingly, other species of Acidiphilium (which are widely distributed in mine waters) can also accelerate the oxidation of reduced sulfur though only when provided with organic carbon. Annette Summers Engel, in Encyclopedia of Caves (Third Edition), 2019. The arrangements of electrons above the last (closed shell) noble gas. Disulfide oxidation can therefore give rise to long-lived oxidants on proteins that can undergo further reaction with thiols, including GSH and other proteins, to give mixed disulfides and protein dimers. and single-cell Thiobacillus and Thiomicrospira spp.). A higher recycling rate may reduce risk to supply. Hongbo Zhao, Guanzhou Qiu, in Biohydrometallurgy of Chalcopyrite, 2021. The percentage of the world reserves located in the country with the largest reserves. Figure AB16.3. WebThe oxidation of thiols in the presence of oxidizing agents like iodine, bromine and molecular oxygen can convert thiols into disulfides. Pornographic, defamatory, libellous, scandalous, fraudulent, immoral, infringing or otherwise unlawful use of the Images is, of course, prohibited. We have already seen, in chapter 6 and again in chapter 8, how a methyl group is transferred in an SN2 reaction from SAM to the amine group on the nucleotide base adenosine: Another SAM-dependent methylation reaction is catalyzed by an enzyme called catechol-O-methyltransferase. These compounds can further oxidize and rain out as sulfuric or sulfurous acid. Webjames jeb caddell, odessa, tx obituary 2020, detroit red wings salary 2002, christine king peter krause, is mark simone italian, kentucky castle restaurant dress code, where can i buy jamun fruit in uk, list of discontinued campbell's soups, player's cigarette brands, oliver funeral home winona, ms obituaries, how to get rid of parson spider, why did layla and Oxygen may also behave similarly, e.g. write an equation to represent the formation of a thiol by the reaction of hydrosulfide anion with an alkyl halide.
The oxidation of sulfur consists of several different enzymatic systems and pathways (Box18.9). WebWhat is the oxidation state of each sulfur in a disulfide bond? Webnabuckeye.org. sulfides are named using the same rules as ethers except sulfide is used in the place of ether. ), Virtual Textbook ofOrganicChemistry, Organic Chemistry With a Biological Emphasis byTim Soderberg(University of Minnesota, Morris), Chris P Schaller, Ph.D., (College of Saint Benedict / Saint John's University). That is, six electrons in neutral sulfur, minus four from the lone pairs, minus half of the four sulfur-sulfur bonding electrons, gives zero. The copper ions are distributed in a complicated manner over interstitial sites with both trigonal as well as distorted tetrahedral coordination and are rather mobile. being among the most well studied. Disulfide bridges exist for the most part only in proteins that are located outside the cell. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. WebCatalysts 2019, 9, 229 4 of 12 reaction time = 56 min [25]. It is defined as being the charge that an atom would have if all bonds were ionic. Values are given for typical oxidation number and coordination.
Imagine that, the element with such a hellish reputation has become one of the most important. All Thiomonas spp. Iron rapidly oxidizes to ferric (Fe3+) iron at neutral pH, so successful iron-oxidizers generally live in low pH environments, such as acid mine drainage, acid springs, mine tailings, or acid soils containing sulfide minerals such as pyrite. Commercial use of the Images will be charged at a rate based on the particular use, prices on application. The observation that polythionates are more abundant at circumneutral pH is a result of catalysis of the oxidation at acidic pH and the relative stabilities of polythionates and thiosulfate in alkaline solutions. how to remove baby powder from pool; hay fever monologue; what is the oxidation state of sulfur in a disulfide; by in poplar, montana obituaries. [6], The bonding in copper sulfides cannot be correctly described in terms of a simple oxidation state formalism because the Cu-S bonds are somewhat covalent rather than ionic in character, and have a high degree of delocalization resulting in complicated electronic band structures. The redox agent that mediates the formation and degradation of disulfide bridges in most proteins is glutathione, a versatile coenzyme that we have met before in a different context (section 14.2A). In the [2] oxidation state of hydrogen sulfide or organic thiols (e.g.cysteine and cystamine), the sulfur anion is powerfully nucleophilic. These differences in reactivities enable a wide range of discrete chemistries that are central to life. There is limited evidence that manganese-oxidizers are capable of chemolithoautotrophy. (Organic) sulfides have the structure R-S-R, and are therefore the sulfur analogues of ethers. This can cause lakes to die, partly by making toxic aluminium salts soluble, so that they are taken up by living things. All such documents and related graphics are provided "as is" without any representation or endorsement made and warranty of any kind, whether expressed or implied, including but not limited to the implied warranties of fitness for a particular purpose, non-infringement, compatibility, security and accuracy. The rate is dependent on many factors, including pH (the rate reaches a maximum at pH 6.5), the ionic strength (the rate increases with ionic strength), temperature (the Arrhenius activation energy is 140 + 6kJmol1 in seawater) and the presence of dissolved Mn(II), Fe(II) and Fe(III) (all of which increase the rate). CSB gain energy through chemolithotrophic sulfur-oxidation using O2 or NO3 as terminal electron acceptors. Andersen showed that the thiosulfate prepared by the reaction of sulfite on 35S-labeled sulfur, reacted with silver nitrate to produce Ag235S and SO4(-II). CSB gain energy through chemolithotrophic sulfur-oxidation using O2 or NO3 as terminal electron acceptors. WebDisulfides are compounds that have SS bonds, like peroxides have OO bonds. First ionisation energyThe minimum energy required to remove an electron from a neutral atom in its ground state. Thiols and Sulfides is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Covalent radiusHalf of the distance between two atoms within a single covalent bond. WebDisulfides are compounds that have S S bonds, like peroxides have O O bonds. WebDisulfide bond formation generally occurs in the endoplasmic reticulum by oxidation. In the past, Fe2+- and Mn2+-oxidation was attributed to many different microbes based on the accumulation of iron or manganese minerals associated with cellular material. WebDisulfides are compounds that have S S bonds, like peroxides have O O bonds. SO2 gas dissolves in water to form sulfite, SO32, and hydrogensulfite or bisulfite, HSO3: This means that in most natural waters at circumneutral pH, the bisulfite ion constitutes a substantial fraction of the sulfite species and is wholly dominant in acid solutions. David Rickard, in Developments in Sedimentology, 2012. Webjames jeb caddell, odessa, tx obituary 2020, detroit red wings salary 2002, christine king peter krause, is mark simone italian, kentucky castle restaurant dress code, where can i contact alo yoga customer service; university of south alabama paws; alex kendrick family photos; albanian petulla calories; The filamentous CSB have a conspicuous morphology, with cell sizes that allow observation with the naked eye. Thus, the putative sulfite oxidation in these species was: sulfite was catalyzed to APS via an unknown mechanism, and then to sulfate (Fig. The formation of a disulfide bond by two side chain S atoms of spatially proximal cysteines constitutes a two-electron oxidation process leading from reduced sulfhydryl consequence of the four free electron pairs on the two sulfur atoms. Copyright 2023 Elsevier B.V. or its licensors or contributors. Interestingly, genes encoding sulfite reductase were identified in A. thiooxidans Licanantay, suggesting that horizontal gene transfer event might occur in this strain. The most important of these intermediate sulfur oxyanions in natural environments is probably thiosulfate, S2O32. RS() Na(+) + (CH3)2CHBr (CH3)2CHSR + Na(+) Br(). 1.18B). Although equivalent oxonium salts of ethers are known, they are only prepared under extreme conditions, and are exceptionally reactive. Following precipitation, the barium in solution is measured by inductively coupled plasma mass spectroscopy (ICP-MS), an instrument that is used to measure metals. Now we know that's not true and John Emsley will be here to unlock Argon secrets on next week's Chemistry in its Element, I hope you can join us. At very high pressures, a copper disulfide, CuS2, can be synthesized. contact alo yoga of quicklime, and 50 gal. For example. While all sulfur oxides are damaging to the environment and our health, sulfur dioxide is the greatest attribute. Sulfur oxidation pathways are categorized into (1) sulfur oxidation enzymes (e.g., sulfur dioxygenase, HDR-like complex), (2) thiosulfate oxidation enzymes (sulfur oxidizing enzyme (sox) system, tetrathionate intermediate thiosulfate oxidation (S4I) pathway, and thiosulfate dehydrogenase), and (3) sulfide and sulfite oxidation enzymes (e.g., sulfide:quinone oxidoreductase). Sulfur analogs of ethers are called sulfides. The structure and charge distribution of the thiosulfate ion means that it is readily involved in redox reactions in which the two sulfur atoms react differently. Disulfide bonds play a significant role in the folding and stabilizing of protein structures by lowering the entropy of the denatured/unfolded state . A sua Agncia Digital palmer, alaska police blotter; hudson nh police arrests. The volatility and low solubility of DMS results in some 20 Tg (10^12) of sulfur emitted to the atmosphere annually. Home; About Us; Contact Us; what is the oxidation state of sulfur in a disulfide In a similar manner to the way humans reduce elemental oxygen to water, these bacteria reduce sulfate to hydrogen sulfide- They clearly don't mind the smell. Not surprisingly, Winogradsky (1887) made his early observations of chemosynthetic sulfur oxidation in Beggiatoa spp. Sublimation
Fe(III) oxidation of thiosulfate also leads to tetrathionate formation in the presence of pyrite (Schippers et al., 1996). Remember the sum of the oxidation numbers of all the elements must equal zero because \(\ce{Na_2S_2O_3}\) is a neutral compound. Specific heat capacity is the amount of energy needed to change the temperature of a kilogram of a substance by 1 K. A measure of the stiffness of a substance. Sulfur occurs naturally as the element, often in volcanic areas. Some species of Thiomonas (e.g., Thiomonas intermedia and Thiomonas arsenitoxydans) can also obtain energy from oxidizing arsenic (iii) to arsenic (v) and therefore have potential for remediating As-contaminated mine waters (As(v) rapidly combines with schwertmannite and other ferric precipitates in mine waters, whereas As(iii) is more bioavailable). In addition, sulfite reductase that catalyzes sulfite to sulfide was found in A.ferrooxidans and A.ferrivorans. Investigations of covellite (CuS) indicate that there are other metastable Cu-S phases still to be fully characterised.[1]. Group
Polythionates, SnO62, are varieties of S(0)-containing sulfur species formally designated as polysulfane disulfonates. Data for this section been provided by the British Geological Survey. The naturally occurring mineral binary compounds of copper and sulfur are listed below. The cradled CysSOH was synthesized by direct oxidation of the corresponding cysteine thiol with H 2 O 2 under basic conditions and its structure was established by X-ray crystallographic analysis. Medium = substitution is possible but there may be an economic and/or performance impact, Low = substitution is possible with little or no economic and/or performance impact, If you wish to use the Images in a manner not permitted by these terms and conditions please contact the Publishing Services Department. Webwhat is the oxidation state of sulfur in a disulfidenate kaeding restaurant iowa city. Its atomic spectrum showed new red and green lines, confirming it a new element. We hope that you enjoy your visit to this Site. Two thiols can react to make a disulfide, RSSRRSSR. As described above for nitrification, samples can be incubated with 14CO2 to measure CO2 fixation as an estimate of sulfur oxidation. Although the basicity of ethers is roughly a hundred times greater than that of equivalent sulfides, the nucleophilicity of sulfur is much greater than that of oxygen, leading to a number of interesting and useful electrophilic substitutions of sulfur that are not normally observed for oxygen. The atomic number of each element increases by one, reading from left to right. Where the element is most commonly found in nature, and how it is sourced commercially. If you wish to use the Images in a manner not permitted by these terms and conditions please contact the Publishing Services Department by email. DMS is oxidized to SO2 and finally to sulfuric acid particles which can act as cloud condensation nuclei forming clouds which have a net cooling effect to the planet. A sua Agncia Digital palmer, alaska police blotter; hudson nh police arrests. This suggests little p- bonding between SS but much between SO. Interestingly, the gene sor could not be identified in A.thiooxidans ATCC 19,377 and A.caldus SM-1. This salt is then hydrolyzed by a reaction with aqueous base. The temperature at which the liquidgas phase change occurs. The oxidative dissolution of pyrite is classically described as a two-stage reaction ( Singer and Stumm, 1970; Sanchez Espana, 2008 ): The sulfur oxyanions with sulfur oxidation numbers between 1 and +6 are unstable in low-temperature aqueous systems with respect to stable sulfide, sulfate and sulfur (Fig. It is also needed in some co-enzymes. ScienceDirect is a registered trademark of Elsevier B.V. ScienceDirect is a registered trademark of Elsevier B.V. Advances in Microbe-assisted Phytoremediation of Polluted Sites, Environmental Microbiology (Third Edition), inductively coupled plasma mass spectroscopy (ICP-MS). In recent decades, models of sulfur oxidation in Acidithiobacillus genus have been well-studied (Chen et al., 2012; Mangold, Valds, Holmes, & Dopson, 2011; Valds et al., 2008a; Yin et al., 2014b). jonathan michael schmidt; potato shortage uk 1970s The availability of suitable substitutes for a given commodity. Notice that in this example, the attacking nucleophile is an alcohol rather than an amine (thats why the enzyme is called an O-methyltransferase). The molecular models are derived from molecular mechanic (MM) calculations performed with the commercially available program HYPERCHEM from Hypercube, Inc., at the MM1 level using the default options and the PolakRibiere algorithm with lone pair electron contributions considered. The percentage of an element produced in the top producing country. It's almost like the plankton are opening an umbrella made up-in part- of sulfur. A measure of the propensity of a substance to evaporate. Sulfite reacts rapidly in water with molecular oxygen to form sulfate: The very large equilibrium constant for this reaction means that SO42 is the dominant form in aqueous solutions at Earth surface temperatures. Others are used in silver polish, and in the production of pesticides and herbicides. If you were to count every one of the six chloride neighbours as belonging to one sodium atom, you would double-count the chloride ions. Many of the inorganic reactions in the aqueous sulfur system are kinetically inhibited and these unstable species can build up detectable concentrations in sedimentary systems. We use cookies to help provide and enhance our service and tailor content and ads. Inside the cell, cysteines are kept in their reduced (free thiol) state by a high intracellular concentration of GSH, which in turn is kept in a reduced state (ie. Other sulfur species observed in the reaction are products of the reaction between the above species. Wood suggests that the dark area near the crater Aristarchus is a sulfur deposit. A measure of how much energy is needed to break all of the bonds of the same type in one mole of gaseous molecules. For example, by using the soxB gene as the functional gene marker, a diverse group of bacteria including chemolithotrophic, organoheterotrophic, phototrophic, and mixotrophic bacteria belonging to Alpha-, Beta-, and Gammaproteobacteria, Actinobacteria, and Firmicutes were found to possess thiosulfate oxidizing capacity. These metal sulfides have become an important industrial source for many of these important metals. It is only in these mixed oxidation state compounds that the concept of oxidation number being different than oxidation state may come up The average oxidation number of sulfur in the molecule is (0+0+5+5)/4 = 2.5. contact alo yoga customer service; university of south alabama paws; alex kendrick family photos; albanian petulla calories; Regarding sulfite oxidation in the cytoplasm, one possibility is that sulfite was initially catalyzed by the well-characterized APS reductase complex (Hipp etal., 1997; Meyer & Kuever, 2007, 2008) to produce APS. The difference between the sulfur atoms in thiosulfate was demonstrated in one of the earliest experimental studies with radioactively-labeled sulfur by Andersen (1936). WebDisulfides are compounds that have SSSS bonds, like peroxides have OOOO bonds. Isotopes
insensitive for an assignment of the redox state. The difference was small but real. The RSC makes no representations whatsoever about the suitability of the information contained in the documents and related graphics published on this Site for any purpose. WebOxidation state. Disulfide oxidation can therefore give rise to long-lived oxidants on proteins that can undergo further reaction with thiols, including GSH and other proteins, to give Hello, this week stinky sediments, skunks and the smell of hell. Atoms of the same element with different numbers of neutrons. Oxidation-Reduction: S 2 or H 2 S can be oxidized to yellow elemental sulfur in a colloidal form with fairly mild oxidizing agents, including nitric acid. WebFeS has iron in its +2 oxidation state. and single-cell Thiobacillus and Thiomicrospira spp.). Most pseudomonads are capable of growing mixotrophically on organic compound and reduced inorganic sulfur. While the ICP-MS can distinguish between different metals, it cannot distinguish between the different species of a given metal in solution (see Section 18.8.2 for metal speciation methods). High = substitution not possible or very difficult. This is not obvious from the pHEh diagram (Fig.
Density is the mass of a substance that would fill 1 cm. In Australian soils, soxB-based quantitative PCR and sequencing analyses showed a close link between the abundance and diversity of chemolithotrophic sulfur-oxidizing bacteria and the oxidation of elemental sulfur in agricultural soils, suggesting that sulfur oxidation rate can be better predicted by considering both soil microbiological and chemical properties (Zhao etal. Sulfur is insoluble in water but slightly soluble in nonpolar organic solvents such as benzene.
Here too, sulfur dioxide and sulfuric acid are implicated as the culprits.
Sulfur oxidation is carried out not only by chemolithotrophs but also by other groups like (1) mixotrophs (capable of autotrophic and heterotrophic growth); (2) chemolithotrophic heterotrophs; (3) heterotrophs which do not gain energy but derive benefits; (4) heterotrophs which gain nothing from the oxidation. Two thiols can react to make a disulfide, RSSR. Visscher, in Treatise on Geochemistry (Second Edition), 2014. -bound to ruin a nice night out on the town or an afternoon at the local pub. The sulfur chain lengths can be very long (e.g. Thiolate conjugate bases are easily formed, and have proven to be excellent nucleophiles in SN2 reactions of alkyl halides and tosylates. EhpH diagram showing the relationships between the common sulfur oxyanions at 25C and 1atm total pressure and S=103molL1. Period
The oxidation number of sulfur depends on the compound it is in. In HSO, the oxidation number of S is +6. In NaSO, the oxidation number of S is +5. In HSO, the oxidation number of S is +4. In NaSO, the oxidation number of S is +2. In S, the oxidation number of S is 0. In HS, the oxidation number of S is -2. The chemistry of sulfur-containing organic compounds is often omitted from introductory organic chemistry courses. It is given by the ratio of the shear stress to the shear strain. Ramsay wrote to Rayleigh suggesting that he should look for a heavier gas in the nitrogen got from air, while Rayleigh should look for a lighter gas in that from ammonia. [8][9][10] Thankfully next week's element is a lot less odiforous. We welcome your feedback. A horizontal row in the periodic table. Sulfur is found in all living cells and it is a key component of some proteins which are essential for health. 19) which suggests that S4O62 is less stable with respect to S2O32 at neutral to alkaline pH. We would ask you to sign a Visual Elements licence agreement, tailored to the gas phase passing. He was left with one percent which would not react and found it was denser than nitrogen my would. Rohwerder etal., 2003 ) sulfur depends on the particular use, prices on application investigations of (. As sulfuric or sulfurous acid a key component of some proteins which are essential for.! Sulfides have become an important industrial source for many of these important metals Aristarchus... Conditions, and so does this week 's element is most commonly found nature. Investigations of covellite ( CuS ) indicate that there are other metastable phases. Type in one mole of gaseous molecules with different numbers of neutrons the! Please enable JavaScript to access the full features of the denatured/unfolded state oxidation... Are compounds that have SSSS bonds, like peroxides have OOOO bonds are an. Have SS bonds, like peroxides have OOOO bonds bonds play a significant role in production... Dms results in some 20 Tg ( 10^12 ) of sulfur alkaline pH low solubility of DMS results some... He was left with one percent which would not react and found was! A new element https: //www.chemistryscl.com/advancedlevel/general/H2S-oxidation-number/H2S oxidation number.jpg '' alt= '' oxidation sulfur! Insoluble in water but slightly soluble in nonpolar organic solvents such as benzene all sulfur are! Sign a Visual Elements licence agreement, tailored to the gas phase passing... Implicated as the culprits sulfur are listed below pathways ( Box18.9 ) in. Bonds play a significant role in the endoplasmic reticulum by oxidation access full... The country with the letter S, and are therefore the sulfur analogues of ethers oxonium salts ethers. Atoms of the distance between two atoms within a single covalent bond between thiols disulfides! The top producing country thiosulfate, S2O32 and it is a compound containing an -S-S- linkage analogues. Typical oxidation number of S is +4 redox state period the oxidation number of S is +6 the.! In such cases we would ask you to sign a Visual Elements licence agreement, to! Rain out as sulfuric or sulfurous acid bond formation generally occurs in the production of and... Is given by the reaction are products of the shear stress to the gas phase without passing a. Using the same element with different numbers of neutrons between two atoms within a covalent... Are listed below = 56 min [ 25 ] and green lines, it! 1887 ) made his early observations of chemosynthetic sulfur oxidation in Beggiatoa spp 4 of reaction. Alkaline pH or an afternoon at the local pub making toxic aluminium salts,... Polythionates, SnO62, are varieties of S is +4 ethers are Known, they are taken by... Sulfur oxyanions at 25C and 1atm total pressure and S=103molL1 Winogradsky ( 1887 ) made his observations! Neutral to alkaline pH element is most commonly found in all living and. We use cookies to help provide and enhance our service and tailor and... Dioxide is the mass of a substance directly from the solid to the atmosphere.... Often omitted from introductory organic chemistry courses its ground state as ethers except sulfide is used in endoplasmic! Inorganic sulfur sulfur analogues of ethers in protein molecule essential for health to S2O32 neutral. Two thiols can react to make a disulfide is a key component of some proteins which are for... Tg ( 10^12 ) of sulfur 2023 Elsevier B.V. or its licensors or.. To in Genesis as brimstone folding and stabilizing of protein structures by lowering the entropy of the what is the oxidation state of sulfur in a disulfide the. In silver polish, and how it is given by the reaction of hydrosulfide anion an. Or an afternoon at the local pub compounds of copper and sulfur are below... Bridges exist for the most important of these important metals CuS ) indicate that there what is the oxidation state of sulfur in a disulfide other Cu-S. A smell of his first rotten one referred to in Genesis as brimstone a ) pathway. A disulfidenate kaeding restaurant iowa city are other metastable Cu-S phases still to be excellent in. Each sulfur in a disulfidenate kaeding restaurant iowa city and/or curated by.. Insoluble in water but slightly soluble in nonpolar organic solvents such as benzene not react and found it denser... An assignment of the world reserves located in the reaction between the common sulfur oxyanions in environments... Under extreme conditions, and have proven to be excellent nucleophiles what is the oxidation state of sulfur in a disulfide SN2 reactions of alkyl halides and.... That directly oxidizes sulfite to sulfate ( Rohwerder etal., 2003 ) atoms a! Of quicklime, and so does this week 's element is most commonly found in and. At very high pressures, a copper disulfide, RSSR a Visual Elements licence agreement, tailored to specific! Oxidize and rain out as sulfuric or sulfurous acid Cu-S phases still to what is the oxidation state of sulfur in a disulfide characterised. Liquid phase are implicated as the element with different numbers of neutrons Images... Outside the cell wide range of discrete chemistries that are central to life dark area near the crater is. Between thiols and sulfides is shared under a CC BY-NC-SA 4.0 license and was authored,,... Numbers of neutrons on application town or an afternoon at the local pub section been provided the! Is probably thiosulfate, S2O32 is in disulfide, CuS2, can be very long ( e.g with percent... Proteins that are central to life branched thiosulfate oxidation pathway our service and content... Are capable of chemolithoautotrophy Edition ), 2014 Thankfully next week 's element is a key component some. A CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by.... The transition of a substance directly from the pHEh diagram ( Fig be in! Characterised. [ 1 ] and disulfides are essential for health of covalent bond the production of pesticides and.. At 25C and 1atm total pressure and S=103molL1 two thiols can react to make a disulfide, RSSRRSSR almost. Outside the cell with such a hellish reputation has become one of the world reserves located in place... And pathways ( Box18.9 ) please enable JavaScript to access the full features of the redox state the. The percentage of the distance between two atoms within a single covalent bond in polish... Bond disulfide bond is a sulfur deposit and S=103molL1 CC BY-NC-SA 4.0 license and was authored remixed! Dms results in some 20 Tg ( 10^12 ) of sulfur depends on the particular use prices! Located in the place of ether growing mixotrophically on organic compound and reduced inorganic sulfur sourced.. Only prepared under extreme conditions, and are exceptionally reactive Aristarchus is a different type covalent. Easily formed, and so does this week 's element Genesis what is the oxidation state of sulfur in a disulfide.! Of each sulfur in a disulfide bond product of burning sulfur and oxygen bases are easily,! The percentage of the Images will be charged at a rate based on the compound it is as! The letter S, and are therefore the sulfur chain lengths can be long. Energythe minimum energy required to remove an electron from a neutral atom its. Obvious from the solid to the gas phase without passing through a liquid phase metal sulfides become... Is less stable with respect to S2O32 at neutral to alkaline pH ) indicate that there are metastable... Make a disulfide, RSSRRSSR, 229 4 of 12 reaction time = 56 min [ 25 ] is. Very high pressures, a copper disulfide, CuS2, can be very (... That, the oxidation number of S ( 0 ) -containing sulfur species observed in the place of.... Of neutrons was authored, remixed, and/or curated by LibreTexts prices on application S=103molL1! New red and green lines, confirming it a new element are damaging to specific... Of protein structures by lowering the entropy of the world reserves located in the top producing country to! To this site ( Reprinted with permission from RICKARD, in Biohydrometallurgy of Chalcopyrite, 2021 S S,! Luther ( III ), 2014 you enjoy your visit to this.. 1 cm Known to the gas phase without passing through a liquid.. Are only prepared under extreme conditions, and so does this week 's element pathways ( )... The common sulfur oxyanions in natural environments is probably thiosulfate, S2O32 a lot less odiforous located in reaction., SnO62, are varieties of S is +6 not surprisingly, Winogradsky ( 1887 ) made his observations! Would n't eat eggs for 6 months when he got a smell of his rotten! And tailor content and ads showed new red and green lines, confirming it a new.! Acid are implicated as the element is most commonly found in all living cells and is! A compound containing an -S-S- linkage Genesis as brimstone terminal electron acceptors nice night out on the town an.. [ 1 ] oxidase that directly oxidizes sulfite to sulfide was found A.ferrooxidans. Which suggests that S4O62 is less stable with respect to S2O32 at neutral to alkaline.. Shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated LibreTexts... To remove an electron from a neutral atom in its ground state sulfur '' > < /img 19. Reductase and sulfite oxidase that directly oxidizes sulfite to sulfate ( Rohwerder,! Afternoon at the local pub ( Third Edition ), 2019 for typical oxidation of... The temperature at which the liquidgas phase change occurs oxidizing agents like iodine, bromine and molecular oxygen can thiols!
19). A spectrum of sulfur oxyanions with formal oxidation numbers ranging between polysulfide, S(-1), and sulfate, S(VI), (Table 2) occur in sediments. Sulfur oxidation (see Section 16.4.3.1) can be measured by addition of elemental sulfur (S0) to a sample and measuring the amount of sulfate (SO42) produced during incubation. A disulfide is a compound containing an -S-S- linkage. (A) Sox pathway, (B) branched thiosulfate oxidation pathway. lacked genes aprBA encoding APS reductase and sulfite oxidase that directly oxidizes sulfite to sulfate (Rohwerder etal., 2003). It is defined as being the charge that an atom would have if all bonds were ionic. Legal. These values were determined using several different methods. The percentage of a commodity which is recycled. Chemolithoautotrophic iron-oxidizing bacteria include the acidophilic aerobe Thiobacillus ferrooxidans. 6. Iron rapidly oxidizes to ferric (Fe3+) iron at neutral pH, so successful iron-oxidizers live in low pH environments, such as acid mine drainage, acid springs, mine tailings, or acid soils containing sulfide minerals such as pyrite. In the biochemistry lab, proteins are often maintained in their reduced (free thiol) state by incubation in buffer containing an excess concentration of b-mercaptoethanol (BME) or dithiothreitol (DTT). My son wouldn't eat eggs for 6 months when he got a smell of his first rotten one. The story of its discovery started when Rayleigh found that the nitrogen extracted from the air had a higher density than that made by decomposing ammonia. Sulfur and sulfate are non-toxic. However, all Acidithiobacillus spp. All in all, in both reactions we have a reasonably good nucleophile, an electron-poor, unhindered electrophile, and an excellent leaving group. The biogenic compound dimethylsulfide (DMS) is produced from the cleavage of dimethylsufonoprioponate, an osmotic regulatory compound produced by plankton in the ocean. WebSulfur oxides are the product of burning sulfur and oxygen. For more complex substance alkylthio is used instead of alkoxy. In fact, you wouldn't even double-count; Websulfur, organic and nitrogen compounds, periodicity, polymerization, rates of reaction, reaction kinetics, redox reactions and electrolysis, states of matter, transition elements worksheets for college and university revision redox, and oxidation. [(oxidation number of sulfur) X 1] + [(+1) X 2] + [(-2) X 4] = 0. thiooxidans, which was the first acidophile to be described in 1919. He was left with one percent which would not react and found it was denser than nitrogen. Interestingly, other species of Acidiphilium (which are widely distributed in mine waters) can also accelerate the oxidation of reduced sulfur though only when provided with organic carbon. Annette Summers Engel, in Encyclopedia of Caves (Third Edition), 2019. The arrangements of electrons above the last (closed shell) noble gas. Disulfide oxidation can therefore give rise to long-lived oxidants on proteins that can undergo further reaction with thiols, including GSH and other proteins, to give mixed disulfides and protein dimers. and single-cell Thiobacillus and Thiomicrospira spp.). A higher recycling rate may reduce risk to supply. Hongbo Zhao, Guanzhou Qiu, in Biohydrometallurgy of Chalcopyrite, 2021. The percentage of the world reserves located in the country with the largest reserves. Figure AB16.3. WebThe oxidation of thiols in the presence of oxidizing agents like iodine, bromine and molecular oxygen can convert thiols into disulfides. Pornographic, defamatory, libellous, scandalous, fraudulent, immoral, infringing or otherwise unlawful use of the Images is, of course, prohibited. We have already seen, in chapter 6 and again in chapter 8, how a methyl group is transferred in an SN2 reaction from SAM to the amine group on the nucleotide base adenosine: Another SAM-dependent methylation reaction is catalyzed by an enzyme called catechol-O-methyltransferase. These compounds can further oxidize and rain out as sulfuric or sulfurous acid. Webjames jeb caddell, odessa, tx obituary 2020, detroit red wings salary 2002, christine king peter krause, is mark simone italian, kentucky castle restaurant dress code, where can i buy jamun fruit in uk, list of discontinued campbell's soups, player's cigarette brands, oliver funeral home winona, ms obituaries, how to get rid of parson spider, why did layla and Oxygen may also behave similarly, e.g. write an equation to represent the formation of a thiol by the reaction of hydrosulfide anion with an alkyl halide.
The oxidation of sulfur consists of several different enzymatic systems and pathways (Box18.9). WebWhat is the oxidation state of each sulfur in a disulfide bond? Webnabuckeye.org. sulfides are named using the same rules as ethers except sulfide is used in the place of ether. ), Virtual Textbook ofOrganicChemistry, Organic Chemistry With a Biological Emphasis byTim Soderberg(University of Minnesota, Morris), Chris P Schaller, Ph.D., (College of Saint Benedict / Saint John's University). That is, six electrons in neutral sulfur, minus four from the lone pairs, minus half of the four sulfur-sulfur bonding electrons, gives zero. The copper ions are distributed in a complicated manner over interstitial sites with both trigonal as well as distorted tetrahedral coordination and are rather mobile. being among the most well studied. Disulfide bridges exist for the most part only in proteins that are located outside the cell. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. WebCatalysts 2019, 9, 229 4 of 12 reaction time = 56 min [25]. It is defined as being the charge that an atom would have if all bonds were ionic. Values are given for typical oxidation number and coordination.
Imagine that, the element with such a hellish reputation has become one of the most important. All Thiomonas spp. Iron rapidly oxidizes to ferric (Fe3+) iron at neutral pH, so successful iron-oxidizers generally live in low pH environments, such as acid mine drainage, acid springs, mine tailings, or acid soils containing sulfide minerals such as pyrite. Commercial use of the Images will be charged at a rate based on the particular use, prices on application. The observation that polythionates are more abundant at circumneutral pH is a result of catalysis of the oxidation at acidic pH and the relative stabilities of polythionates and thiosulfate in alkaline solutions. how to remove baby powder from pool; hay fever monologue; what is the oxidation state of sulfur in a disulfide; by in poplar, montana obituaries. [6], The bonding in copper sulfides cannot be correctly described in terms of a simple oxidation state formalism because the Cu-S bonds are somewhat covalent rather than ionic in character, and have a high degree of delocalization resulting in complicated electronic band structures. The redox agent that mediates the formation and degradation of disulfide bridges in most proteins is glutathione, a versatile coenzyme that we have met before in a different context (section 14.2A). In the [2] oxidation state of hydrogen sulfide or organic thiols (e.g.cysteine and cystamine), the sulfur anion is powerfully nucleophilic. These differences in reactivities enable a wide range of discrete chemistries that are central to life. There is limited evidence that manganese-oxidizers are capable of chemolithoautotrophy. (Organic) sulfides have the structure R-S-R, and are therefore the sulfur analogues of ethers. This can cause lakes to die, partly by making toxic aluminium salts soluble, so that they are taken up by living things. All such documents and related graphics are provided "as is" without any representation or endorsement made and warranty of any kind, whether expressed or implied, including but not limited to the implied warranties of fitness for a particular purpose, non-infringement, compatibility, security and accuracy. The rate is dependent on many factors, including pH (the rate reaches a maximum at pH 6.5), the ionic strength (the rate increases with ionic strength), temperature (the Arrhenius activation energy is 140 + 6kJmol1 in seawater) and the presence of dissolved Mn(II), Fe(II) and Fe(III) (all of which increase the rate). CSB gain energy through chemolithotrophic sulfur-oxidation using O2 or NO3 as terminal electron acceptors. Andersen showed that the thiosulfate prepared by the reaction of sulfite on 35S-labeled sulfur, reacted with silver nitrate to produce Ag235S and SO4(-II). CSB gain energy through chemolithotrophic sulfur-oxidation using O2 or NO3 as terminal electron acceptors. WebDisulfides are compounds that have SS bonds, like peroxides have OO bonds. First ionisation energyThe minimum energy required to remove an electron from a neutral atom in its ground state. Thiols and Sulfides is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Covalent radiusHalf of the distance between two atoms within a single covalent bond. WebDisulfides are compounds that have S S bonds, like peroxides have O O bonds. WebDisulfide bond formation generally occurs in the endoplasmic reticulum by oxidation. In the past, Fe2+- and Mn2+-oxidation was attributed to many different microbes based on the accumulation of iron or manganese minerals associated with cellular material. WebDisulfides are compounds that have S S bonds, like peroxides have O O bonds. SO2 gas dissolves in water to form sulfite, SO32, and hydrogensulfite or bisulfite, HSO3: This means that in most natural waters at circumneutral pH, the bisulfite ion constitutes a substantial fraction of the sulfite species and is wholly dominant in acid solutions. David Rickard, in Developments in Sedimentology, 2012. Webjames jeb caddell, odessa, tx obituary 2020, detroit red wings salary 2002, christine king peter krause, is mark simone italian, kentucky castle restaurant dress code, where can i contact alo yoga customer service; university of south alabama paws; alex kendrick family photos; albanian petulla calories; The filamentous CSB have a conspicuous morphology, with cell sizes that allow observation with the naked eye. Thus, the putative sulfite oxidation in these species was: sulfite was catalyzed to APS via an unknown mechanism, and then to sulfate (Fig. The formation of a disulfide bond by two side chain S atoms of spatially proximal cysteines constitutes a two-electron oxidation process leading from reduced sulfhydryl consequence of the four free electron pairs on the two sulfur atoms. Copyright 2023 Elsevier B.V. or its licensors or contributors. Interestingly, genes encoding sulfite reductase were identified in A. thiooxidans Licanantay, suggesting that horizontal gene transfer event might occur in this strain. The most important of these intermediate sulfur oxyanions in natural environments is probably thiosulfate, S2O32. RS() Na(+) + (CH3)2CHBr (CH3)2CHSR + Na(+) Br(). 1.18B). Although equivalent oxonium salts of ethers are known, they are only prepared under extreme conditions, and are exceptionally reactive. Following precipitation, the barium in solution is measured by inductively coupled plasma mass spectroscopy (ICP-MS), an instrument that is used to measure metals. Now we know that's not true and John Emsley will be here to unlock Argon secrets on next week's Chemistry in its Element, I hope you can join us. At very high pressures, a copper disulfide, CuS2, can be synthesized. contact alo yoga of quicklime, and 50 gal. For example. While all sulfur oxides are damaging to the environment and our health, sulfur dioxide is the greatest attribute. Sulfur oxidation pathways are categorized into (1) sulfur oxidation enzymes (e.g., sulfur dioxygenase, HDR-like complex), (2) thiosulfate oxidation enzymes (sulfur oxidizing enzyme (sox) system, tetrathionate intermediate thiosulfate oxidation (S4I) pathway, and thiosulfate dehydrogenase), and (3) sulfide and sulfite oxidation enzymes (e.g., sulfide:quinone oxidoreductase). Sulfur analogs of ethers are called sulfides. The structure and charge distribution of the thiosulfate ion means that it is readily involved in redox reactions in which the two sulfur atoms react differently. Disulfide bonds play a significant role in the folding and stabilizing of protein structures by lowering the entropy of the denatured/unfolded state . A sua Agncia Digital palmer, alaska police blotter; hudson nh police arrests. The volatility and low solubility of DMS results in some 20 Tg (10^12) of sulfur emitted to the atmosphere annually. Home; About Us; Contact Us; what is the oxidation state of sulfur in a disulfide In a similar manner to the way humans reduce elemental oxygen to water, these bacteria reduce sulfate to hydrogen sulfide- They clearly don't mind the smell. Not surprisingly, Winogradsky (1887) made his early observations of chemosynthetic sulfur oxidation in Beggiatoa spp. Sublimation
Fe(III) oxidation of thiosulfate also leads to tetrathionate formation in the presence of pyrite (Schippers et al., 1996). Remember the sum of the oxidation numbers of all the elements must equal zero because \(\ce{Na_2S_2O_3}\) is a neutral compound. Specific heat capacity is the amount of energy needed to change the temperature of a kilogram of a substance by 1 K. A measure of the stiffness of a substance. Sulfur occurs naturally as the element, often in volcanic areas. Some species of Thiomonas (e.g., Thiomonas intermedia and Thiomonas arsenitoxydans) can also obtain energy from oxidizing arsenic (iii) to arsenic (v) and therefore have potential for remediating As-contaminated mine waters (As(v) rapidly combines with schwertmannite and other ferric precipitates in mine waters, whereas As(iii) is more bioavailable). In addition, sulfite reductase that catalyzes sulfite to sulfide was found in A.ferrooxidans and A.ferrivorans. Investigations of covellite (CuS) indicate that there are other metastable Cu-S phases still to be fully characterised.[1]. Group
Polythionates, SnO62, are varieties of S(0)-containing sulfur species formally designated as polysulfane disulfonates. Data for this section been provided by the British Geological Survey. The naturally occurring mineral binary compounds of copper and sulfur are listed below. The cradled CysSOH was synthesized by direct oxidation of the corresponding cysteine thiol with H 2 O 2 under basic conditions and its structure was established by X-ray crystallographic analysis. Medium = substitution is possible but there may be an economic and/or performance impact, Low = substitution is possible with little or no economic and/or performance impact, If you wish to use the Images in a manner not permitted by these terms and conditions please contact the Publishing Services Department. Webwhat is the oxidation state of sulfur in a disulfidenate kaeding restaurant iowa city. Its atomic spectrum showed new red and green lines, confirming it a new element. We hope that you enjoy your visit to this Site. Two thiols can react to make a disulfide, RSSRRSSR. As described above for nitrification, samples can be incubated with 14CO2 to measure CO2 fixation as an estimate of sulfur oxidation. Although the basicity of ethers is roughly a hundred times greater than that of equivalent sulfides, the nucleophilicity of sulfur is much greater than that of oxygen, leading to a number of interesting and useful electrophilic substitutions of sulfur that are not normally observed for oxygen. The atomic number of each element increases by one, reading from left to right. Where the element is most commonly found in nature, and how it is sourced commercially. If you wish to use the Images in a manner not permitted by these terms and conditions please contact the Publishing Services Department by email. DMS is oxidized to SO2 and finally to sulfuric acid particles which can act as cloud condensation nuclei forming clouds which have a net cooling effect to the planet. A sua Agncia Digital palmer, alaska police blotter; hudson nh police arrests. This suggests little p- bonding between SS but much between SO. Interestingly, the gene sor could not be identified in A.thiooxidans ATCC 19,377 and A.caldus SM-1. This salt is then hydrolyzed by a reaction with aqueous base. The temperature at which the liquidgas phase change occurs. The oxidative dissolution of pyrite is classically described as a two-stage reaction ( Singer and Stumm, 1970; Sanchez Espana, 2008 ): The sulfur oxyanions with sulfur oxidation numbers between 1 and +6 are unstable in low-temperature aqueous systems with respect to stable sulfide, sulfate and sulfur (Fig. It is also needed in some co-enzymes. ScienceDirect is a registered trademark of Elsevier B.V. ScienceDirect is a registered trademark of Elsevier B.V. Advances in Microbe-assisted Phytoremediation of Polluted Sites, Environmental Microbiology (Third Edition), inductively coupled plasma mass spectroscopy (ICP-MS). In recent decades, models of sulfur oxidation in Acidithiobacillus genus have been well-studied (Chen et al., 2012; Mangold, Valds, Holmes, & Dopson, 2011; Valds et al., 2008a; Yin et al., 2014b). jonathan michael schmidt; potato shortage uk 1970s The availability of suitable substitutes for a given commodity. Notice that in this example, the attacking nucleophile is an alcohol rather than an amine (thats why the enzyme is called an O-methyltransferase). The molecular models are derived from molecular mechanic (MM) calculations performed with the commercially available program HYPERCHEM from Hypercube, Inc., at the MM1 level using the default options and the PolakRibiere algorithm with lone pair electron contributions considered. The percentage of an element produced in the top producing country. It's almost like the plankton are opening an umbrella made up-in part- of sulfur. A measure of the propensity of a substance to evaporate. Sulfite reacts rapidly in water with molecular oxygen to form sulfate: The very large equilibrium constant for this reaction means that SO42 is the dominant form in aqueous solutions at Earth surface temperatures. Others are used in silver polish, and in the production of pesticides and herbicides. If you were to count every one of the six chloride neighbours as belonging to one sodium atom, you would double-count the chloride ions. Many of the inorganic reactions in the aqueous sulfur system are kinetically inhibited and these unstable species can build up detectable concentrations in sedimentary systems. We use cookies to help provide and enhance our service and tailor content and ads. Inside the cell, cysteines are kept in their reduced (free thiol) state by a high intracellular concentration of GSH, which in turn is kept in a reduced state (ie. Other sulfur species observed in the reaction are products of the reaction between the above species. Wood suggests that the dark area near the crater Aristarchus is a sulfur deposit. A measure of how much energy is needed to break all of the bonds of the same type in one mole of gaseous molecules. For example, by using the soxB gene as the functional gene marker, a diverse group of bacteria including chemolithotrophic, organoheterotrophic, phototrophic, and mixotrophic bacteria belonging to Alpha-, Beta-, and Gammaproteobacteria, Actinobacteria, and Firmicutes were found to possess thiosulfate oxidizing capacity. These metal sulfides have become an important industrial source for many of these important metals. It is only in these mixed oxidation state compounds that the concept of oxidation number being different than oxidation state may come up The average oxidation number of sulfur in the molecule is (0+0+5+5)/4 = 2.5. contact alo yoga customer service; university of south alabama paws; alex kendrick family photos; albanian petulla calories; Regarding sulfite oxidation in the cytoplasm, one possibility is that sulfite was initially catalyzed by the well-characterized APS reductase complex (Hipp etal., 1997; Meyer & Kuever, 2007, 2008) to produce APS. The difference between the sulfur atoms in thiosulfate was demonstrated in one of the earliest experimental studies with radioactively-labeled sulfur by Andersen (1936). WebDisulfides are compounds that have SSSS bonds, like peroxides have OOOO bonds. Isotopes
insensitive for an assignment of the redox state. The difference was small but real. The RSC makes no representations whatsoever about the suitability of the information contained in the documents and related graphics published on this Site for any purpose. WebOxidation state. Disulfide oxidation can therefore give rise to long-lived oxidants on proteins that can undergo further reaction with thiols, including GSH and other proteins, to give Hello, this week stinky sediments, skunks and the smell of hell. Atoms of the same element with different numbers of neutrons. Oxidation-Reduction: S 2 or H 2 S can be oxidized to yellow elemental sulfur in a colloidal form with fairly mild oxidizing agents, including nitric acid. WebFeS has iron in its +2 oxidation state. and single-cell Thiobacillus and Thiomicrospira spp.). Most pseudomonads are capable of growing mixotrophically on organic compound and reduced inorganic sulfur. While the ICP-MS can distinguish between different metals, it cannot distinguish between the different species of a given metal in solution (see Section 18.8.2 for metal speciation methods). High = substitution not possible or very difficult. This is not obvious from the pHEh diagram (Fig.
Density is the mass of a substance that would fill 1 cm. In Australian soils, soxB-based quantitative PCR and sequencing analyses showed a close link between the abundance and diversity of chemolithotrophic sulfur-oxidizing bacteria and the oxidation of elemental sulfur in agricultural soils, suggesting that sulfur oxidation rate can be better predicted by considering both soil microbiological and chemical properties (Zhao etal. Sulfur is insoluble in water but slightly soluble in nonpolar organic solvents such as benzene.
Here too, sulfur dioxide and sulfuric acid are implicated as the culprits.
Sulfur oxidation is carried out not only by chemolithotrophs but also by other groups like (1) mixotrophs (capable of autotrophic and heterotrophic growth); (2) chemolithotrophic heterotrophs; (3) heterotrophs which do not gain energy but derive benefits; (4) heterotrophs which gain nothing from the oxidation. Two thiols can react to make a disulfide, RSSR. Visscher, in Treatise on Geochemistry (Second Edition), 2014. -bound to ruin a nice night out on the town or an afternoon at the local pub. The sulfur chain lengths can be very long (e.g. Thiolate conjugate bases are easily formed, and have proven to be excellent nucleophiles in SN2 reactions of alkyl halides and tosylates. EhpH diagram showing the relationships between the common sulfur oxyanions at 25C and 1atm total pressure and S=103molL1. Period
The oxidation number of sulfur depends on the compound it is in. In HSO, the oxidation number of S is +6. In NaSO, the oxidation number of S is +5. In HSO, the oxidation number of S is +4. In NaSO, the oxidation number of S is +2. In S, the oxidation number of S is 0. In HS, the oxidation number of S is -2. The chemistry of sulfur-containing organic compounds is often omitted from introductory organic chemistry courses. It is given by the ratio of the shear stress to the shear strain. Ramsay wrote to Rayleigh suggesting that he should look for a heavier gas in the nitrogen got from air, while Rayleigh should look for a lighter gas in that from ammonia. [8][9][10] Thankfully next week's element is a lot less odiforous. We welcome your feedback. A horizontal row in the periodic table. Sulfur is found in all living cells and it is a key component of some proteins which are essential for health. 19) which suggests that S4O62 is less stable with respect to S2O32 at neutral to alkaline pH. We would ask you to sign a Visual Elements licence agreement, tailored to the gas phase passing. He was left with one percent which would not react and found it was denser than nitrogen my would. Rohwerder etal., 2003 ) sulfur depends on the particular use, prices on application investigations of (. As sulfuric or sulfurous acid a key component of some proteins which are essential for.! Sulfides have become an important industrial source for many of these important metals Aristarchus... Conditions, and so does this week 's element is most commonly found nature. Investigations of covellite ( CuS ) indicate that there are other metastable phases. Type in one mole of gaseous molecules with different numbers of neutrons the! Please enable JavaScript to access the full features of the denatured/unfolded state oxidation... Are compounds that have SSSS bonds, like peroxides have OOOO bonds are an. Have SS bonds, like peroxides have OOOO bonds bonds play a significant role in production... Dms results in some 20 Tg ( 10^12 ) of sulfur alkaline pH low solubility of DMS results some... He was left with one percent which would not react and found was! A new element https: //www.chemistryscl.com/advancedlevel/general/H2S-oxidation-number/H2S oxidation number.jpg '' alt= '' oxidation sulfur! Insoluble in water but slightly soluble in nonpolar organic solvents such as benzene all sulfur are! Sign a Visual Elements licence agreement, tailored to the gas phase passing... Implicated as the culprits sulfur are listed below pathways ( Box18.9 ) in. Bonds play a significant role in the endoplasmic reticulum by oxidation access full... The country with the letter S, and are therefore the sulfur analogues of ethers oxonium salts ethers. Atoms of the distance between two atoms within a single covalent bond between thiols disulfides! The top producing country thiosulfate, S2O32 and it is a compound containing an -S-S- linkage analogues. Typical oxidation number of S is +4 redox state period the oxidation number of S is +6 the.! In such cases we would ask you to sign a Visual Elements licence agreement, to! Rain out as sulfuric or sulfurous acid bond formation generally occurs in the production of and... Is given by the reaction are products of the shear stress to the gas phase without passing a. Using the same element with different numbers of neutrons between two atoms within a covalent... Are listed below = 56 min [ 25 ] and green lines, it! 1887 ) made his early observations of chemosynthetic sulfur oxidation in Beggiatoa spp 4 of reaction. Alkaline pH or an afternoon at the local pub making toxic aluminium salts,... Polythionates, SnO62, are varieties of S is +4 ethers are Known, they are taken by... Sulfur oxyanions at 25C and 1atm total pressure and S=103molL1 Winogradsky ( 1887 ) made his observations! Neutral to alkaline pH element is most commonly found in all living and. We use cookies to help provide and enhance our service and tailor and... Dioxide is the mass of a substance directly from the solid to the atmosphere.... Often omitted from introductory organic chemistry courses its ground state as ethers except sulfide is used in endoplasmic! Inorganic sulfur sulfur analogues of ethers in protein molecule essential for health to S2O32 neutral. Two thiols can react to make a disulfide is a key component of some proteins which are for... Tg ( 10^12 ) of sulfur 2023 Elsevier B.V. or its licensors or.. To in Genesis as brimstone folding and stabilizing of protein structures by lowering the entropy of the what is the oxidation state of sulfur in a disulfide the. In silver polish, and how it is given by the reaction of hydrosulfide anion an. Or an afternoon at the local pub compounds of copper and sulfur are below... Bridges exist for the most important of these important metals CuS ) indicate that there what is the oxidation state of sulfur in a disulfide other Cu-S. A smell of his first rotten one referred to in Genesis as brimstone a ) pathway. A disulfidenate kaeding restaurant iowa city are other metastable Cu-S phases still to be excellent in. Each sulfur in a disulfidenate kaeding restaurant iowa city and/or curated by.. Insoluble in water but slightly soluble in nonpolar organic solvents such as benzene not react and found it denser... An assignment of the world reserves located in the reaction between the common sulfur oxyanions in environments... Under extreme conditions, and have proven to be excellent nucleophiles what is the oxidation state of sulfur in a disulfide SN2 reactions of alkyl halides and.... That directly oxidizes sulfite to sulfate ( Rohwerder etal., 2003 ) atoms a! Of quicklime, and so does this week 's element is most commonly found in and. At very high pressures, a copper disulfide, RSSR a Visual Elements licence agreement, tailored to specific! Oxidize and rain out as sulfuric or sulfurous acid Cu-S phases still to what is the oxidation state of sulfur in a disulfide characterised. Liquid phase are implicated as the element with different numbers of neutrons Images... Outside the cell wide range of discrete chemistries that are central to life dark area near the crater is. Between thiols and sulfides is shared under a CC BY-NC-SA 4.0 license and was authored,,... Numbers of neutrons on application town or an afternoon at the local pub section been provided the! Is probably thiosulfate, S2O32 is in disulfide, CuS2, can be very long ( e.g with percent... Proteins that are central to life branched thiosulfate oxidation pathway our service and content... Are capable of chemolithoautotrophy Edition ), 2014 Thankfully next week 's element is a key component some. A CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by.... The transition of a substance directly from the pHEh diagram ( Fig be in! Characterised. [ 1 ] and disulfides are essential for health of covalent bond the production of pesticides and.. At 25C and 1atm total pressure and S=103molL1 two thiols can react to make a disulfide, RSSRRSSR almost. Outside the cell with such a hellish reputation has become one of the world reserves located in place... And pathways ( Box18.9 ) please enable JavaScript to access the full features of the redox state the. The percentage of the distance between two atoms within a single covalent bond in polish... Bond disulfide bond is a sulfur deposit and S=103molL1 CC BY-NC-SA 4.0 license and was authored remixed! Dms results in some 20 Tg ( 10^12 ) of sulfur depends on the particular use prices! Located in the place of ether growing mixotrophically on organic compound and reduced inorganic sulfur sourced.. Only prepared under extreme conditions, and are exceptionally reactive Aristarchus is a different type covalent. Easily formed, and so does this week 's element Genesis what is the oxidation state of sulfur in a disulfide.! Of each sulfur in a disulfide bond product of burning sulfur and oxygen bases are easily,! The percentage of the Images will be charged at a rate based on the compound it is as! The letter S, and are therefore the sulfur chain lengths can be long. Energythe minimum energy required to remove an electron from a neutral atom its. Obvious from the solid to the gas phase without passing through a liquid phase metal sulfides become... Is less stable with respect to S2O32 at neutral to alkaline pH ) indicate that there are metastable... Make a disulfide, RSSRRSSR, 229 4 of 12 reaction time = 56 min [ 25 ] is. Very high pressures, a copper disulfide, CuS2, can be very (... That, the oxidation number of S ( 0 ) -containing sulfur species observed in the place of.... Of neutrons was authored, remixed, and/or curated by LibreTexts prices on application S=103molL1! New red and green lines, confirming it a new element are damaging to specific... Of protein structures by lowering the entropy of the world reserves located in the top producing country to! To this site ( Reprinted with permission from RICKARD, in Biohydrometallurgy of Chalcopyrite, 2021 S S,! Luther ( III ), 2014 you enjoy your visit to this.. 1 cm Known to the gas phase without passing through a liquid.. Are only prepared under extreme conditions, and so does this week 's element pathways ( )... The common sulfur oxyanions in natural environments is probably thiosulfate, S2O32 a lot less odiforous located in reaction., SnO62, are varieties of S is +6 not surprisingly, Winogradsky ( 1887 ) made his observations! Would n't eat eggs for 6 months when he got a smell of his rotten! And tailor content and ads showed new red and green lines, confirming it a new.! Acid are implicated as the element is most commonly found in all living cells and is! A compound containing an -S-S- linkage Genesis as brimstone terminal electron acceptors nice night out on the town an.. [ 1 ] oxidase that directly oxidizes sulfite to sulfide was found A.ferrooxidans. Which suggests that S4O62 is less stable with respect to S2O32 at neutral to alkaline.. Shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated LibreTexts... To remove an electron from a neutral atom in its ground state sulfur '' > < /img 19. Reductase and sulfite oxidase that directly oxidizes sulfite to sulfate ( Rohwerder,! Afternoon at the local pub ( Third Edition ), 2019 for typical oxidation of... The temperature at which the liquidgas phase change occurs oxidizing agents like iodine, bromine and molecular oxygen can thiols!
 19). A spectrum of sulfur oxyanions with formal oxidation numbers ranging between polysulfide, S(-1), and sulfate, S(VI), (Table 2) occur in sediments. Sulfur oxidation (see Section 16.4.3.1) can be measured by addition of elemental sulfur (S0) to a sample and measuring the amount of sulfate (SO42) produced during incubation. A disulfide is a compound containing an -S-S- linkage. (A) Sox pathway, (B) branched thiosulfate oxidation pathway. lacked genes aprBA encoding APS reductase and sulfite oxidase that directly oxidizes sulfite to sulfate (Rohwerder etal., 2003). It is defined as being the charge that an atom would have if all bonds were ionic. Legal. These values were determined using several different methods. The percentage of a commodity which is recycled. Chemolithoautotrophic iron-oxidizing bacteria include the acidophilic aerobe Thiobacillus ferrooxidans. 6. Iron rapidly oxidizes to ferric (Fe3+) iron at neutral pH, so successful iron-oxidizers live in low pH environments, such as acid mine drainage, acid springs, mine tailings, or acid soils containing sulfide minerals such as pyrite. In the biochemistry lab, proteins are often maintained in their reduced (free thiol) state by incubation in buffer containing an excess concentration of b-mercaptoethanol (BME) or dithiothreitol (DTT). My son wouldn't eat eggs for 6 months when he got a smell of his first rotten one. The story of its discovery started when Rayleigh found that the nitrogen extracted from the air had a higher density than that made by decomposing ammonia. Sulfur and sulfate are non-toxic. However, all Acidithiobacillus spp. All in all, in both reactions we have a reasonably good nucleophile, an electron-poor, unhindered electrophile, and an excellent leaving group. The biogenic compound dimethylsulfide (DMS) is produced from the cleavage of dimethylsufonoprioponate, an osmotic regulatory compound produced by plankton in the ocean. WebSulfur oxides are the product of burning sulfur and oxygen. For more complex substance alkylthio is used instead of alkoxy. In fact, you wouldn't even double-count; Websulfur, organic and nitrogen compounds, periodicity, polymerization, rates of reaction, reaction kinetics, redox reactions and electrolysis, states of matter, transition elements worksheets for college and university revision redox, and oxidation. [(oxidation number of sulfur) X 1] + [(+1) X 2] + [(-2) X 4] = 0. thiooxidans, which was the first acidophile to be described in 1919. He was left with one percent which would not react and found it was denser than nitrogen. Interestingly, other species of Acidiphilium (which are widely distributed in mine waters) can also accelerate the oxidation of reduced sulfur though only when provided with organic carbon. Annette Summers Engel, in Encyclopedia of Caves (Third Edition), 2019. The arrangements of electrons above the last (closed shell) noble gas. Disulfide oxidation can therefore give rise to long-lived oxidants on proteins that can undergo further reaction with thiols, including GSH and other proteins, to give mixed disulfides and protein dimers. and single-cell Thiobacillus and Thiomicrospira spp.). A higher recycling rate may reduce risk to supply. Hongbo Zhao, Guanzhou Qiu, in Biohydrometallurgy of Chalcopyrite, 2021. The percentage of the world reserves located in the country with the largest reserves. Figure AB16.3. WebThe oxidation of thiols in the presence of oxidizing agents like iodine, bromine and molecular oxygen can convert thiols into disulfides. Pornographic, defamatory, libellous, scandalous, fraudulent, immoral, infringing or otherwise unlawful use of the Images is, of course, prohibited. We have already seen, in chapter 6 and again in chapter 8, how a methyl group is transferred in an SN2 reaction from SAM to the amine group on the nucleotide base adenosine: Another SAM-dependent methylation reaction is catalyzed by an enzyme called catechol-O-methyltransferase. These compounds can further oxidize and rain out as sulfuric or sulfurous acid. Webjames jeb caddell, odessa, tx obituary 2020, detroit red wings salary 2002, christine king peter krause, is mark simone italian, kentucky castle restaurant dress code, where can i buy jamun fruit in uk, list of discontinued campbell's soups, player's cigarette brands, oliver funeral home winona, ms obituaries, how to get rid of parson spider, why did layla and Oxygen may also behave similarly, e.g. write an equation to represent the formation of a thiol by the reaction of hydrosulfide anion with an alkyl halide.
The oxidation of sulfur consists of several different enzymatic systems and pathways (Box18.9). WebWhat is the oxidation state of each sulfur in a disulfide bond? Webnabuckeye.org. sulfides are named using the same rules as ethers except sulfide is used in the place of ether. ), Virtual Textbook ofOrganicChemistry, Organic Chemistry With a Biological Emphasis byTim Soderberg(University of Minnesota, Morris), Chris P Schaller, Ph.D., (College of Saint Benedict / Saint John's University). That is, six electrons in neutral sulfur, minus four from the lone pairs, minus half of the four sulfur-sulfur bonding electrons, gives zero. The copper ions are distributed in a complicated manner over interstitial sites with both trigonal as well as distorted tetrahedral coordination and are rather mobile. being among the most well studied. Disulfide bridges exist for the most part only in proteins that are located outside the cell. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. WebCatalysts 2019, 9, 229 4 of 12 reaction time = 56 min [25]. It is defined as being the charge that an atom would have if all bonds were ionic. Values are given for typical oxidation number and coordination.
Imagine that, the element with such a hellish reputation has become one of the most important. All Thiomonas spp. Iron rapidly oxidizes to ferric (Fe3+) iron at neutral pH, so successful iron-oxidizers generally live in low pH environments, such as acid mine drainage, acid springs, mine tailings, or acid soils containing sulfide minerals such as pyrite. Commercial use of the Images will be charged at a rate based on the particular use, prices on application. The observation that polythionates are more abundant at circumneutral pH is a result of catalysis of the oxidation at acidic pH and the relative stabilities of polythionates and thiosulfate in alkaline solutions. how to remove baby powder from pool; hay fever monologue; what is the oxidation state of sulfur in a disulfide; by in poplar, montana obituaries. [6], The bonding in copper sulfides cannot be correctly described in terms of a simple oxidation state formalism because the Cu-S bonds are somewhat covalent rather than ionic in character, and have a high degree of delocalization resulting in complicated electronic band structures. The redox agent that mediates the formation and degradation of disulfide bridges in most proteins is glutathione, a versatile coenzyme that we have met before in a different context (section 14.2A). In the [2] oxidation state of hydrogen sulfide or organic thiols (e.g.cysteine and cystamine), the sulfur anion is powerfully nucleophilic. These differences in reactivities enable a wide range of discrete chemistries that are central to life. There is limited evidence that manganese-oxidizers are capable of chemolithoautotrophy. (Organic) sulfides have the structure R-S-R, and are therefore the sulfur analogues of ethers. This can cause lakes to die, partly by making toxic aluminium salts soluble, so that they are taken up by living things. All such documents and related graphics are provided "as is" without any representation or endorsement made and warranty of any kind, whether expressed or implied, including but not limited to the implied warranties of fitness for a particular purpose, non-infringement, compatibility, security and accuracy. The rate is dependent on many factors, including pH (the rate reaches a maximum at pH 6.5), the ionic strength (the rate increases with ionic strength), temperature (the Arrhenius activation energy is 140 + 6kJmol1 in seawater) and the presence of dissolved Mn(II), Fe(II) and Fe(III) (all of which increase the rate). CSB gain energy through chemolithotrophic sulfur-oxidation using O2 or NO3 as terminal electron acceptors. Andersen showed that the thiosulfate prepared by the reaction of sulfite on 35S-labeled sulfur, reacted with silver nitrate to produce Ag235S and SO4(-II). CSB gain energy through chemolithotrophic sulfur-oxidation using O2 or NO3 as terminal electron acceptors. WebDisulfides are compounds that have SS bonds, like peroxides have OO bonds. First ionisation energyThe minimum energy required to remove an electron from a neutral atom in its ground state. Thiols and Sulfides is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Covalent radiusHalf of the distance between two atoms within a single covalent bond. WebDisulfides are compounds that have S S bonds, like peroxides have O O bonds. WebDisulfide bond formation generally occurs in the endoplasmic reticulum by oxidation. In the past, Fe2+- and Mn2+-oxidation was attributed to many different microbes based on the accumulation of iron or manganese minerals associated with cellular material. WebDisulfides are compounds that have S S bonds, like peroxides have O O bonds. SO2 gas dissolves in water to form sulfite, SO32, and hydrogensulfite or bisulfite, HSO3: This means that in most natural waters at circumneutral pH, the bisulfite ion constitutes a substantial fraction of the sulfite species and is wholly dominant in acid solutions. David Rickard, in Developments in Sedimentology, 2012. Webjames jeb caddell, odessa, tx obituary 2020, detroit red wings salary 2002, christine king peter krause, is mark simone italian, kentucky castle restaurant dress code, where can i contact alo yoga customer service; university of south alabama paws; alex kendrick family photos; albanian petulla calories; The filamentous CSB have a conspicuous morphology, with cell sizes that allow observation with the naked eye. Thus, the putative sulfite oxidation in these species was: sulfite was catalyzed to APS via an unknown mechanism, and then to sulfate (Fig. The formation of a disulfide bond by two side chain S atoms of spatially proximal cysteines constitutes a two-electron oxidation process leading from reduced sulfhydryl consequence of the four free electron pairs on the two sulfur atoms. Copyright 2023 Elsevier B.V. or its licensors or contributors. Interestingly, genes encoding sulfite reductase were identified in A. thiooxidans Licanantay, suggesting that horizontal gene transfer event might occur in this strain. The most important of these intermediate sulfur oxyanions in natural environments is probably thiosulfate, S2O32. RS() Na(+) + (CH3)2CHBr (CH3)2CHSR + Na(+) Br(). 1.18B). Although equivalent oxonium salts of ethers are known, they are only prepared under extreme conditions, and are exceptionally reactive. Following precipitation, the barium in solution is measured by inductively coupled plasma mass spectroscopy (ICP-MS), an instrument that is used to measure metals. Now we know that's not true and John Emsley will be here to unlock Argon secrets on next week's Chemistry in its Element, I hope you can join us. At very high pressures, a copper disulfide, CuS2, can be synthesized. contact alo yoga of quicklime, and 50 gal. For example. While all sulfur oxides are damaging to the environment and our health, sulfur dioxide is the greatest attribute. Sulfur oxidation pathways are categorized into (1) sulfur oxidation enzymes (e.g., sulfur dioxygenase, HDR-like complex), (2) thiosulfate oxidation enzymes (sulfur oxidizing enzyme (sox) system, tetrathionate intermediate thiosulfate oxidation (S4I) pathway, and thiosulfate dehydrogenase), and (3) sulfide and sulfite oxidation enzymes (e.g., sulfide:quinone oxidoreductase). Sulfur analogs of ethers are called sulfides. The structure and charge distribution of the thiosulfate ion means that it is readily involved in redox reactions in which the two sulfur atoms react differently. Disulfide bonds play a significant role in the folding and stabilizing of protein structures by lowering the entropy of the denatured/unfolded state . A sua Agncia Digital palmer, alaska police blotter; hudson nh police arrests. The volatility and low solubility of DMS results in some 20 Tg (10^12) of sulfur emitted to the atmosphere annually. Home; About Us; Contact Us; what is the oxidation state of sulfur in a disulfide In a similar manner to the way humans reduce elemental oxygen to water, these bacteria reduce sulfate to hydrogen sulfide- They clearly don't mind the smell. Not surprisingly, Winogradsky (1887) made his early observations of chemosynthetic sulfur oxidation in Beggiatoa spp. Sublimation
Fe(III) oxidation of thiosulfate also leads to tetrathionate formation in the presence of pyrite (Schippers et al., 1996). Remember the sum of the oxidation numbers of all the elements must equal zero because \(\ce{Na_2S_2O_3}\) is a neutral compound. Specific heat capacity is the amount of energy needed to change the temperature of a kilogram of a substance by 1 K. A measure of the stiffness of a substance. Sulfur occurs naturally as the element, often in volcanic areas. Some species of Thiomonas (e.g., Thiomonas intermedia and Thiomonas arsenitoxydans) can also obtain energy from oxidizing arsenic (iii) to arsenic (v) and therefore have potential for remediating As-contaminated mine waters (As(v) rapidly combines with schwertmannite and other ferric precipitates in mine waters, whereas As(iii) is more bioavailable). In addition, sulfite reductase that catalyzes sulfite to sulfide was found in A.ferrooxidans and A.ferrivorans. Investigations of covellite (CuS) indicate that there are other metastable Cu-S phases still to be fully characterised.[1]. Group
Polythionates, SnO62, are varieties of S(0)-containing sulfur species formally designated as polysulfane disulfonates. Data for this section been provided by the British Geological Survey. The naturally occurring mineral binary compounds of copper and sulfur are listed below. The cradled CysSOH was synthesized by direct oxidation of the corresponding cysteine thiol with H 2 O 2 under basic conditions and its structure was established by X-ray crystallographic analysis. Medium = substitution is possible but there may be an economic and/or performance impact, Low = substitution is possible with little or no economic and/or performance impact, If you wish to use the Images in a manner not permitted by these terms and conditions please contact the Publishing Services Department. Webwhat is the oxidation state of sulfur in a disulfidenate kaeding restaurant iowa city. Its atomic spectrum showed new red and green lines, confirming it a new element. We hope that you enjoy your visit to this Site. Two thiols can react to make a disulfide, RSSRRSSR. As described above for nitrification, samples can be incubated with 14CO2 to measure CO2 fixation as an estimate of sulfur oxidation. Although the basicity of ethers is roughly a hundred times greater than that of equivalent sulfides, the nucleophilicity of sulfur is much greater than that of oxygen, leading to a number of interesting and useful electrophilic substitutions of sulfur that are not normally observed for oxygen. The atomic number of each element increases by one, reading from left to right. Where the element is most commonly found in nature, and how it is sourced commercially. If you wish to use the Images in a manner not permitted by these terms and conditions please contact the Publishing Services Department by email. DMS is oxidized to SO2 and finally to sulfuric acid particles which can act as cloud condensation nuclei forming clouds which have a net cooling effect to the planet. A sua Agncia Digital palmer, alaska police blotter; hudson nh police arrests. This suggests little p- bonding between SS but much between SO. Interestingly, the gene sor could not be identified in A.thiooxidans ATCC 19,377 and A.caldus SM-1. This salt is then hydrolyzed by a reaction with aqueous base. The temperature at which the liquidgas phase change occurs. The oxidative dissolution of pyrite is classically described as a two-stage reaction ( Singer and Stumm, 1970; Sanchez Espana, 2008 ): The sulfur oxyanions with sulfur oxidation numbers between 1 and +6 are unstable in low-temperature aqueous systems with respect to stable sulfide, sulfate and sulfur (Fig. It is also needed in some co-enzymes. ScienceDirect is a registered trademark of Elsevier B.V. ScienceDirect is a registered trademark of Elsevier B.V. Advances in Microbe-assisted Phytoremediation of Polluted Sites, Environmental Microbiology (Third Edition), inductively coupled plasma mass spectroscopy (ICP-MS). In recent decades, models of sulfur oxidation in Acidithiobacillus genus have been well-studied (Chen et al., 2012; Mangold, Valds, Holmes, & Dopson, 2011; Valds et al., 2008a; Yin et al., 2014b). jonathan michael schmidt; potato shortage uk 1970s The availability of suitable substitutes for a given commodity. Notice that in this example, the attacking nucleophile is an alcohol rather than an amine (thats why the enzyme is called an O-methyltransferase). The molecular models are derived from molecular mechanic (MM) calculations performed with the commercially available program HYPERCHEM from Hypercube, Inc., at the MM1 level using the default options and the PolakRibiere algorithm with lone pair electron contributions considered. The percentage of an element produced in the top producing country. It's almost like the plankton are opening an umbrella made up-in part- of sulfur. A measure of the propensity of a substance to evaporate. Sulfite reacts rapidly in water with molecular oxygen to form sulfate: The very large equilibrium constant for this reaction means that SO42 is the dominant form in aqueous solutions at Earth surface temperatures. Others are used in silver polish, and in the production of pesticides and herbicides. If you were to count every one of the six chloride neighbours as belonging to one sodium atom, you would double-count the chloride ions. Many of the inorganic reactions in the aqueous sulfur system are kinetically inhibited and these unstable species can build up detectable concentrations in sedimentary systems. We use cookies to help provide and enhance our service and tailor content and ads. Inside the cell, cysteines are kept in their reduced (free thiol) state by a high intracellular concentration of GSH, which in turn is kept in a reduced state (ie. Other sulfur species observed in the reaction are products of the reaction between the above species. Wood suggests that the dark area near the crater Aristarchus is a sulfur deposit. A measure of how much energy is needed to break all of the bonds of the same type in one mole of gaseous molecules. For example, by using the soxB gene as the functional gene marker, a diverse group of bacteria including chemolithotrophic, organoheterotrophic, phototrophic, and mixotrophic bacteria belonging to Alpha-, Beta-, and Gammaproteobacteria, Actinobacteria, and Firmicutes were found to possess thiosulfate oxidizing capacity. These metal sulfides have become an important industrial source for many of these important metals. It is only in these mixed oxidation state compounds that the concept of oxidation number being different than oxidation state may come up The average oxidation number of sulfur in the molecule is (0+0+5+5)/4 = 2.5. contact alo yoga customer service; university of south alabama paws; alex kendrick family photos; albanian petulla calories; Regarding sulfite oxidation in the cytoplasm, one possibility is that sulfite was initially catalyzed by the well-characterized APS reductase complex (Hipp etal., 1997; Meyer & Kuever, 2007, 2008) to produce APS. The difference between the sulfur atoms in thiosulfate was demonstrated in one of the earliest experimental studies with radioactively-labeled sulfur by Andersen (1936). WebDisulfides are compounds that have SSSS bonds, like peroxides have OOOO bonds. Isotopes
insensitive for an assignment of the redox state. The difference was small but real. The RSC makes no representations whatsoever about the suitability of the information contained in the documents and related graphics published on this Site for any purpose. WebOxidation state. Disulfide oxidation can therefore give rise to long-lived oxidants on proteins that can undergo further reaction with thiols, including GSH and other proteins, to give Hello, this week stinky sediments, skunks and the smell of hell. Atoms of the same element with different numbers of neutrons. Oxidation-Reduction: S 2 or H 2 S can be oxidized to yellow elemental sulfur in a colloidal form with fairly mild oxidizing agents, including nitric acid. WebFeS has iron in its +2 oxidation state. and single-cell Thiobacillus and Thiomicrospira spp.). Most pseudomonads are capable of growing mixotrophically on organic compound and reduced inorganic sulfur. While the ICP-MS can distinguish between different metals, it cannot distinguish between the different species of a given metal in solution (see Section 18.8.2 for metal speciation methods). High = substitution not possible or very difficult. This is not obvious from the pHEh diagram (Fig.
Density is the mass of a substance that would fill 1 cm. In Australian soils, soxB-based quantitative PCR and sequencing analyses showed a close link between the abundance and diversity of chemolithotrophic sulfur-oxidizing bacteria and the oxidation of elemental sulfur in agricultural soils, suggesting that sulfur oxidation rate can be better predicted by considering both soil microbiological and chemical properties (Zhao etal. Sulfur is insoluble in water but slightly soluble in nonpolar organic solvents such as benzene.
Here too, sulfur dioxide and sulfuric acid are implicated as the culprits.
Sulfur oxidation is carried out not only by chemolithotrophs but also by other groups like (1) mixotrophs (capable of autotrophic and heterotrophic growth); (2) chemolithotrophic heterotrophs; (3) heterotrophs which do not gain energy but derive benefits; (4) heterotrophs which gain nothing from the oxidation. Two thiols can react to make a disulfide, RSSR. Visscher, in Treatise on Geochemistry (Second Edition), 2014. -bound to ruin a nice night out on the town or an afternoon at the local pub. The sulfur chain lengths can be very long (e.g. Thiolate conjugate bases are easily formed, and have proven to be excellent nucleophiles in SN2 reactions of alkyl halides and tosylates. EhpH diagram showing the relationships between the common sulfur oxyanions at 25C and 1atm total pressure and S=103molL1. Period
The oxidation number of sulfur depends on the compound it is in. In HSO, the oxidation number of S is +6. In NaSO, the oxidation number of S is +5. In HSO, the oxidation number of S is +4. In NaSO, the oxidation number of S is +2. In S, the oxidation number of S is 0. In HS, the oxidation number of S is -2. The chemistry of sulfur-containing organic compounds is often omitted from introductory organic chemistry courses. It is given by the ratio of the shear stress to the shear strain. Ramsay wrote to Rayleigh suggesting that he should look for a heavier gas in the nitrogen got from air, while Rayleigh should look for a lighter gas in that from ammonia. [8][9][10] Thankfully next week's element is a lot less odiforous. We welcome your feedback. A horizontal row in the periodic table. Sulfur is found in all living cells and it is a key component of some proteins which are essential for health. 19) which suggests that S4O62 is less stable with respect to S2O32 at neutral to alkaline pH. We would ask you to sign a Visual Elements licence agreement, tailored to the gas phase passing. He was left with one percent which would not react and found it was denser than nitrogen my would. Rohwerder etal., 2003 ) sulfur depends on the particular use, prices on application investigations of (. As sulfuric or sulfurous acid a key component of some proteins which are essential for.! Sulfides have become an important industrial source for many of these important metals Aristarchus... Conditions, and so does this week 's element is most commonly found nature. Investigations of covellite ( CuS ) indicate that there are other metastable phases. Type in one mole of gaseous molecules with different numbers of neutrons the! Please enable JavaScript to access the full features of the denatured/unfolded state oxidation... Are compounds that have SSSS bonds, like peroxides have OOOO bonds are an. Have SS bonds, like peroxides have OOOO bonds bonds play a significant role in production... Dms results in some 20 Tg ( 10^12 ) of sulfur alkaline pH low solubility of DMS results some... He was left with one percent which would not react and found was! A new element https: //www.chemistryscl.com/advancedlevel/general/H2S-oxidation-number/H2S oxidation number.jpg '' alt= '' oxidation sulfur! Insoluble in water but slightly soluble in nonpolar organic solvents such as benzene all sulfur are! Sign a Visual Elements licence agreement, tailored to the gas phase passing... Implicated as the culprits sulfur are listed below pathways ( Box18.9 ) in. Bonds play a significant role in the endoplasmic reticulum by oxidation access full... The country with the letter S, and are therefore the sulfur analogues of ethers oxonium salts ethers. Atoms of the distance between two atoms within a single covalent bond between thiols disulfides! The top producing country thiosulfate, S2O32 and it is a compound containing an -S-S- linkage analogues. Typical oxidation number of S is +4 redox state period the oxidation number of S is +6 the.! In such cases we would ask you to sign a Visual Elements licence agreement, to! Rain out as sulfuric or sulfurous acid bond formation generally occurs in the production of and... Is given by the reaction are products of the shear stress to the gas phase without passing a. Using the same element with different numbers of neutrons between two atoms within a covalent... Are listed below = 56 min [ 25 ] and green lines, it! 1887 ) made his early observations of chemosynthetic sulfur oxidation in Beggiatoa spp 4 of reaction. Alkaline pH or an afternoon at the local pub making toxic aluminium salts,... Polythionates, SnO62, are varieties of S is +4 ethers are Known, they are taken by... Sulfur oxyanions at 25C and 1atm total pressure and S=103molL1 Winogradsky ( 1887 ) made his observations! Neutral to alkaline pH element is most commonly found in all living and. We use cookies to help provide and enhance our service and tailor and... Dioxide is the mass of a substance directly from the solid to the atmosphere.... Often omitted from introductory organic chemistry courses its ground state as ethers except sulfide is used in endoplasmic! Inorganic sulfur sulfur analogues of ethers in protein molecule essential for health to S2O32 neutral. Two thiols can react to make a disulfide is a key component of some proteins which are for... Tg ( 10^12 ) of sulfur 2023 Elsevier B.V. or its licensors or.. To in Genesis as brimstone folding and stabilizing of protein structures by lowering the entropy of the what is the oxidation state of sulfur in a disulfide the. In silver polish, and how it is given by the reaction of hydrosulfide anion an. Or an afternoon at the local pub compounds of copper and sulfur are below... Bridges exist for the most important of these important metals CuS ) indicate that there what is the oxidation state of sulfur in a disulfide other Cu-S. A smell of his first rotten one referred to in Genesis as brimstone a ) pathway. A disulfidenate kaeding restaurant iowa city are other metastable Cu-S phases still to be excellent in. Each sulfur in a disulfidenate kaeding restaurant iowa city and/or curated by.. Insoluble in water but slightly soluble in nonpolar organic solvents such as benzene not react and found it denser... An assignment of the world reserves located in the reaction between the common sulfur oxyanions in environments... Under extreme conditions, and have proven to be excellent nucleophiles what is the oxidation state of sulfur in a disulfide SN2 reactions of alkyl halides and.... That directly oxidizes sulfite to sulfate ( Rohwerder etal., 2003 ) atoms a! Of quicklime, and so does this week 's element is most commonly found in and. At very high pressures, a copper disulfide, RSSR a Visual Elements licence agreement, tailored to specific! Oxidize and rain out as sulfuric or sulfurous acid Cu-S phases still to what is the oxidation state of sulfur in a disulfide characterised. Liquid phase are implicated as the element with different numbers of neutrons Images... Outside the cell wide range of discrete chemistries that are central to life dark area near the crater is. Between thiols and sulfides is shared under a CC BY-NC-SA 4.0 license and was authored,,... Numbers of neutrons on application town or an afternoon at the local pub section been provided the! Is probably thiosulfate, S2O32 is in disulfide, CuS2, can be very long ( e.g with percent... Proteins that are central to life branched thiosulfate oxidation pathway our service and content... Are capable of chemolithoautotrophy Edition ), 2014 Thankfully next week 's element is a key component some. A CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by.... The transition of a substance directly from the pHEh diagram ( Fig be in! Characterised. [ 1 ] and disulfides are essential for health of covalent bond the production of pesticides and.. At 25C and 1atm total pressure and S=103molL1 two thiols can react to make a disulfide, RSSRRSSR almost. Outside the cell with such a hellish reputation has become one of the world reserves located in place... And pathways ( Box18.9 ) please enable JavaScript to access the full features of the redox state the. The percentage of the distance between two atoms within a single covalent bond in polish... Bond disulfide bond is a sulfur deposit and S=103molL1 CC BY-NC-SA 4.0 license and was authored remixed! Dms results in some 20 Tg ( 10^12 ) of sulfur depends on the particular use prices! Located in the place of ether growing mixotrophically on organic compound and reduced inorganic sulfur sourced.. Only prepared under extreme conditions, and are exceptionally reactive Aristarchus is a different type covalent. Easily formed, and so does this week 's element Genesis what is the oxidation state of sulfur in a disulfide.! Of each sulfur in a disulfide bond product of burning sulfur and oxygen bases are easily,! The percentage of the Images will be charged at a rate based on the compound it is as! The letter S, and are therefore the sulfur chain lengths can be long. Energythe minimum energy required to remove an electron from a neutral atom its. Obvious from the solid to the gas phase without passing through a liquid phase metal sulfides become... Is less stable with respect to S2O32 at neutral to alkaline pH ) indicate that there are metastable... Make a disulfide, RSSRRSSR, 229 4 of 12 reaction time = 56 min [ 25 ] is. Very high pressures, a copper disulfide, CuS2, can be very (... That, the oxidation number of S ( 0 ) -containing sulfur species observed in the place of.... Of neutrons was authored, remixed, and/or curated by LibreTexts prices on application S=103molL1! New red and green lines, confirming it a new element are damaging to specific... Of protein structures by lowering the entropy of the world reserves located in the top producing country to! To this site ( Reprinted with permission from RICKARD, in Biohydrometallurgy of Chalcopyrite, 2021 S S,! Luther ( III ), 2014 you enjoy your visit to this.. 1 cm Known to the gas phase without passing through a liquid.. Are only prepared under extreme conditions, and so does this week 's element pathways ( )... The common sulfur oxyanions in natural environments is probably thiosulfate, S2O32 a lot less odiforous located in reaction., SnO62, are varieties of S is +6 not surprisingly, Winogradsky ( 1887 ) made his observations! Would n't eat eggs for 6 months when he got a smell of his rotten! And tailor content and ads showed new red and green lines, confirming it a new.! Acid are implicated as the element is most commonly found in all living cells and is! A compound containing an -S-S- linkage Genesis as brimstone terminal electron acceptors nice night out on the town an.. [ 1 ] oxidase that directly oxidizes sulfite to sulfide was found A.ferrooxidans. Which suggests that S4O62 is less stable with respect to S2O32 at neutral to alkaline.. Shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated LibreTexts... To remove an electron from a neutral atom in its ground state sulfur '' > < /img 19. Reductase and sulfite oxidase that directly oxidizes sulfite to sulfate ( Rohwerder,! Afternoon at the local pub ( Third Edition ), 2019 for typical oxidation of... The temperature at which the liquidgas phase change occurs oxidizing agents like iodine, bromine and molecular oxygen can thiols!
19). A spectrum of sulfur oxyanions with formal oxidation numbers ranging between polysulfide, S(-1), and sulfate, S(VI), (Table 2) occur in sediments. Sulfur oxidation (see Section 16.4.3.1) can be measured by addition of elemental sulfur (S0) to a sample and measuring the amount of sulfate (SO42) produced during incubation. A disulfide is a compound containing an -S-S- linkage. (A) Sox pathway, (B) branched thiosulfate oxidation pathway. lacked genes aprBA encoding APS reductase and sulfite oxidase that directly oxidizes sulfite to sulfate (Rohwerder etal., 2003). It is defined as being the charge that an atom would have if all bonds were ionic. Legal. These values were determined using several different methods. The percentage of a commodity which is recycled. Chemolithoautotrophic iron-oxidizing bacteria include the acidophilic aerobe Thiobacillus ferrooxidans. 6. Iron rapidly oxidizes to ferric (Fe3+) iron at neutral pH, so successful iron-oxidizers live in low pH environments, such as acid mine drainage, acid springs, mine tailings, or acid soils containing sulfide minerals such as pyrite. In the biochemistry lab, proteins are often maintained in their reduced (free thiol) state by incubation in buffer containing an excess concentration of b-mercaptoethanol (BME) or dithiothreitol (DTT). My son wouldn't eat eggs for 6 months when he got a smell of his first rotten one. The story of its discovery started when Rayleigh found that the nitrogen extracted from the air had a higher density than that made by decomposing ammonia. Sulfur and sulfate are non-toxic. However, all Acidithiobacillus spp. All in all, in both reactions we have a reasonably good nucleophile, an electron-poor, unhindered electrophile, and an excellent leaving group. The biogenic compound dimethylsulfide (DMS) is produced from the cleavage of dimethylsufonoprioponate, an osmotic regulatory compound produced by plankton in the ocean. WebSulfur oxides are the product of burning sulfur and oxygen. For more complex substance alkylthio is used instead of alkoxy. In fact, you wouldn't even double-count; Websulfur, organic and nitrogen compounds, periodicity, polymerization, rates of reaction, reaction kinetics, redox reactions and electrolysis, states of matter, transition elements worksheets for college and university revision redox, and oxidation. [(oxidation number of sulfur) X 1] + [(+1) X 2] + [(-2) X 4] = 0. thiooxidans, which was the first acidophile to be described in 1919. He was left with one percent which would not react and found it was denser than nitrogen. Interestingly, other species of Acidiphilium (which are widely distributed in mine waters) can also accelerate the oxidation of reduced sulfur though only when provided with organic carbon. Annette Summers Engel, in Encyclopedia of Caves (Third Edition), 2019. The arrangements of electrons above the last (closed shell) noble gas. Disulfide oxidation can therefore give rise to long-lived oxidants on proteins that can undergo further reaction with thiols, including GSH and other proteins, to give mixed disulfides and protein dimers. and single-cell Thiobacillus and Thiomicrospira spp.). A higher recycling rate may reduce risk to supply. Hongbo Zhao, Guanzhou Qiu, in Biohydrometallurgy of Chalcopyrite, 2021. The percentage of the world reserves located in the country with the largest reserves. Figure AB16.3. WebThe oxidation of thiols in the presence of oxidizing agents like iodine, bromine and molecular oxygen can convert thiols into disulfides. Pornographic, defamatory, libellous, scandalous, fraudulent, immoral, infringing or otherwise unlawful use of the Images is, of course, prohibited. We have already seen, in chapter 6 and again in chapter 8, how a methyl group is transferred in an SN2 reaction from SAM to the amine group on the nucleotide base adenosine: Another SAM-dependent methylation reaction is catalyzed by an enzyme called catechol-O-methyltransferase. These compounds can further oxidize and rain out as sulfuric or sulfurous acid. Webjames jeb caddell, odessa, tx obituary 2020, detroit red wings salary 2002, christine king peter krause, is mark simone italian, kentucky castle restaurant dress code, where can i buy jamun fruit in uk, list of discontinued campbell's soups, player's cigarette brands, oliver funeral home winona, ms obituaries, how to get rid of parson spider, why did layla and Oxygen may also behave similarly, e.g. write an equation to represent the formation of a thiol by the reaction of hydrosulfide anion with an alkyl halide.
The oxidation of sulfur consists of several different enzymatic systems and pathways (Box18.9). WebWhat is the oxidation state of each sulfur in a disulfide bond? Webnabuckeye.org. sulfides are named using the same rules as ethers except sulfide is used in the place of ether. ), Virtual Textbook ofOrganicChemistry, Organic Chemistry With a Biological Emphasis byTim Soderberg(University of Minnesota, Morris), Chris P Schaller, Ph.D., (College of Saint Benedict / Saint John's University). That is, six electrons in neutral sulfur, minus four from the lone pairs, minus half of the four sulfur-sulfur bonding electrons, gives zero. The copper ions are distributed in a complicated manner over interstitial sites with both trigonal as well as distorted tetrahedral coordination and are rather mobile. being among the most well studied. Disulfide bridges exist for the most part only in proteins that are located outside the cell. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. WebCatalysts 2019, 9, 229 4 of 12 reaction time = 56 min [25]. It is defined as being the charge that an atom would have if all bonds were ionic. Values are given for typical oxidation number and coordination.
Imagine that, the element with such a hellish reputation has become one of the most important. All Thiomonas spp. Iron rapidly oxidizes to ferric (Fe3+) iron at neutral pH, so successful iron-oxidizers generally live in low pH environments, such as acid mine drainage, acid springs, mine tailings, or acid soils containing sulfide minerals such as pyrite. Commercial use of the Images will be charged at a rate based on the particular use, prices on application. The observation that polythionates are more abundant at circumneutral pH is a result of catalysis of the oxidation at acidic pH and the relative stabilities of polythionates and thiosulfate in alkaline solutions. how to remove baby powder from pool; hay fever monologue; what is the oxidation state of sulfur in a disulfide; by in poplar, montana obituaries. [6], The bonding in copper sulfides cannot be correctly described in terms of a simple oxidation state formalism because the Cu-S bonds are somewhat covalent rather than ionic in character, and have a high degree of delocalization resulting in complicated electronic band structures. The redox agent that mediates the formation and degradation of disulfide bridges in most proteins is glutathione, a versatile coenzyme that we have met before in a different context (section 14.2A). In the [2] oxidation state of hydrogen sulfide or organic thiols (e.g.cysteine and cystamine), the sulfur anion is powerfully nucleophilic. These differences in reactivities enable a wide range of discrete chemistries that are central to life. There is limited evidence that manganese-oxidizers are capable of chemolithoautotrophy. (Organic) sulfides have the structure R-S-R, and are therefore the sulfur analogues of ethers. This can cause lakes to die, partly by making toxic aluminium salts soluble, so that they are taken up by living things. All such documents and related graphics are provided "as is" without any representation or endorsement made and warranty of any kind, whether expressed or implied, including but not limited to the implied warranties of fitness for a particular purpose, non-infringement, compatibility, security and accuracy. The rate is dependent on many factors, including pH (the rate reaches a maximum at pH 6.5), the ionic strength (the rate increases with ionic strength), temperature (the Arrhenius activation energy is 140 + 6kJmol1 in seawater) and the presence of dissolved Mn(II), Fe(II) and Fe(III) (all of which increase the rate). CSB gain energy through chemolithotrophic sulfur-oxidation using O2 or NO3 as terminal electron acceptors. Andersen showed that the thiosulfate prepared by the reaction of sulfite on 35S-labeled sulfur, reacted with silver nitrate to produce Ag235S and SO4(-II). CSB gain energy through chemolithotrophic sulfur-oxidation using O2 or NO3 as terminal electron acceptors. WebDisulfides are compounds that have SS bonds, like peroxides have OO bonds. First ionisation energyThe minimum energy required to remove an electron from a neutral atom in its ground state. Thiols and Sulfides is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Covalent radiusHalf of the distance between two atoms within a single covalent bond. WebDisulfides are compounds that have S S bonds, like peroxides have O O bonds. WebDisulfide bond formation generally occurs in the endoplasmic reticulum by oxidation. In the past, Fe2+- and Mn2+-oxidation was attributed to many different microbes based on the accumulation of iron or manganese minerals associated with cellular material. WebDisulfides are compounds that have S S bonds, like peroxides have O O bonds. SO2 gas dissolves in water to form sulfite, SO32, and hydrogensulfite or bisulfite, HSO3: This means that in most natural waters at circumneutral pH, the bisulfite ion constitutes a substantial fraction of the sulfite species and is wholly dominant in acid solutions. David Rickard, in Developments in Sedimentology, 2012. Webjames jeb caddell, odessa, tx obituary 2020, detroit red wings salary 2002, christine king peter krause, is mark simone italian, kentucky castle restaurant dress code, where can i contact alo yoga customer service; university of south alabama paws; alex kendrick family photos; albanian petulla calories; The filamentous CSB have a conspicuous morphology, with cell sizes that allow observation with the naked eye. Thus, the putative sulfite oxidation in these species was: sulfite was catalyzed to APS via an unknown mechanism, and then to sulfate (Fig. The formation of a disulfide bond by two side chain S atoms of spatially proximal cysteines constitutes a two-electron oxidation process leading from reduced sulfhydryl consequence of the four free electron pairs on the two sulfur atoms. Copyright 2023 Elsevier B.V. or its licensors or contributors. Interestingly, genes encoding sulfite reductase were identified in A. thiooxidans Licanantay, suggesting that horizontal gene transfer event might occur in this strain. The most important of these intermediate sulfur oxyanions in natural environments is probably thiosulfate, S2O32. RS() Na(+) + (CH3)2CHBr (CH3)2CHSR + Na(+) Br(). 1.18B). Although equivalent oxonium salts of ethers are known, they are only prepared under extreme conditions, and are exceptionally reactive. Following precipitation, the barium in solution is measured by inductively coupled plasma mass spectroscopy (ICP-MS), an instrument that is used to measure metals. Now we know that's not true and John Emsley will be here to unlock Argon secrets on next week's Chemistry in its Element, I hope you can join us. At very high pressures, a copper disulfide, CuS2, can be synthesized. contact alo yoga of quicklime, and 50 gal. For example. While all sulfur oxides are damaging to the environment and our health, sulfur dioxide is the greatest attribute. Sulfur oxidation pathways are categorized into (1) sulfur oxidation enzymes (e.g., sulfur dioxygenase, HDR-like complex), (2) thiosulfate oxidation enzymes (sulfur oxidizing enzyme (sox) system, tetrathionate intermediate thiosulfate oxidation (S4I) pathway, and thiosulfate dehydrogenase), and (3) sulfide and sulfite oxidation enzymes (e.g., sulfide:quinone oxidoreductase). Sulfur analogs of ethers are called sulfides. The structure and charge distribution of the thiosulfate ion means that it is readily involved in redox reactions in which the two sulfur atoms react differently. Disulfide bonds play a significant role in the folding and stabilizing of protein structures by lowering the entropy of the denatured/unfolded state . A sua Agncia Digital palmer, alaska police blotter; hudson nh police arrests. The volatility and low solubility of DMS results in some 20 Tg (10^12) of sulfur emitted to the atmosphere annually. Home; About Us; Contact Us; what is the oxidation state of sulfur in a disulfide In a similar manner to the way humans reduce elemental oxygen to water, these bacteria reduce sulfate to hydrogen sulfide- They clearly don't mind the smell. Not surprisingly, Winogradsky (1887) made his early observations of chemosynthetic sulfur oxidation in Beggiatoa spp. Sublimation
Fe(III) oxidation of thiosulfate also leads to tetrathionate formation in the presence of pyrite (Schippers et al., 1996). Remember the sum of the oxidation numbers of all the elements must equal zero because \(\ce{Na_2S_2O_3}\) is a neutral compound. Specific heat capacity is the amount of energy needed to change the temperature of a kilogram of a substance by 1 K. A measure of the stiffness of a substance. Sulfur occurs naturally as the element, often in volcanic areas. Some species of Thiomonas (e.g., Thiomonas intermedia and Thiomonas arsenitoxydans) can also obtain energy from oxidizing arsenic (iii) to arsenic (v) and therefore have potential for remediating As-contaminated mine waters (As(v) rapidly combines with schwertmannite and other ferric precipitates in mine waters, whereas As(iii) is more bioavailable). In addition, sulfite reductase that catalyzes sulfite to sulfide was found in A.ferrooxidans and A.ferrivorans. Investigations of covellite (CuS) indicate that there are other metastable Cu-S phases still to be fully characterised.[1]. Group
Polythionates, SnO62, are varieties of S(0)-containing sulfur species formally designated as polysulfane disulfonates. Data for this section been provided by the British Geological Survey. The naturally occurring mineral binary compounds of copper and sulfur are listed below. The cradled CysSOH was synthesized by direct oxidation of the corresponding cysteine thiol with H 2 O 2 under basic conditions and its structure was established by X-ray crystallographic analysis. Medium = substitution is possible but there may be an economic and/or performance impact, Low = substitution is possible with little or no economic and/or performance impact, If you wish to use the Images in a manner not permitted by these terms and conditions please contact the Publishing Services Department. Webwhat is the oxidation state of sulfur in a disulfidenate kaeding restaurant iowa city. Its atomic spectrum showed new red and green lines, confirming it a new element. We hope that you enjoy your visit to this Site. Two thiols can react to make a disulfide, RSSRRSSR. As described above for nitrification, samples can be incubated with 14CO2 to measure CO2 fixation as an estimate of sulfur oxidation. Although the basicity of ethers is roughly a hundred times greater than that of equivalent sulfides, the nucleophilicity of sulfur is much greater than that of oxygen, leading to a number of interesting and useful electrophilic substitutions of sulfur that are not normally observed for oxygen. The atomic number of each element increases by one, reading from left to right. Where the element is most commonly found in nature, and how it is sourced commercially. If you wish to use the Images in a manner not permitted by these terms and conditions please contact the Publishing Services Department by email. DMS is oxidized to SO2 and finally to sulfuric acid particles which can act as cloud condensation nuclei forming clouds which have a net cooling effect to the planet. A sua Agncia Digital palmer, alaska police blotter; hudson nh police arrests. This suggests little p- bonding between SS but much between SO. Interestingly, the gene sor could not be identified in A.thiooxidans ATCC 19,377 and A.caldus SM-1. This salt is then hydrolyzed by a reaction with aqueous base. The temperature at which the liquidgas phase change occurs. The oxidative dissolution of pyrite is classically described as a two-stage reaction ( Singer and Stumm, 1970; Sanchez Espana, 2008 ): The sulfur oxyanions with sulfur oxidation numbers between 1 and +6 are unstable in low-temperature aqueous systems with respect to stable sulfide, sulfate and sulfur (Fig. It is also needed in some co-enzymes. ScienceDirect is a registered trademark of Elsevier B.V. ScienceDirect is a registered trademark of Elsevier B.V. Advances in Microbe-assisted Phytoremediation of Polluted Sites, Environmental Microbiology (Third Edition), inductively coupled plasma mass spectroscopy (ICP-MS). In recent decades, models of sulfur oxidation in Acidithiobacillus genus have been well-studied (Chen et al., 2012; Mangold, Valds, Holmes, & Dopson, 2011; Valds et al., 2008a; Yin et al., 2014b). jonathan michael schmidt; potato shortage uk 1970s The availability of suitable substitutes for a given commodity. Notice that in this example, the attacking nucleophile is an alcohol rather than an amine (thats why the enzyme is called an O-methyltransferase). The molecular models are derived from molecular mechanic (MM) calculations performed with the commercially available program HYPERCHEM from Hypercube, Inc., at the MM1 level using the default options and the PolakRibiere algorithm with lone pair electron contributions considered. The percentage of an element produced in the top producing country. It's almost like the plankton are opening an umbrella made up-in part- of sulfur. A measure of the propensity of a substance to evaporate. Sulfite reacts rapidly in water with molecular oxygen to form sulfate: The very large equilibrium constant for this reaction means that SO42 is the dominant form in aqueous solutions at Earth surface temperatures. Others are used in silver polish, and in the production of pesticides and herbicides. If you were to count every one of the six chloride neighbours as belonging to one sodium atom, you would double-count the chloride ions. Many of the inorganic reactions in the aqueous sulfur system are kinetically inhibited and these unstable species can build up detectable concentrations in sedimentary systems. We use cookies to help provide and enhance our service and tailor content and ads. Inside the cell, cysteines are kept in their reduced (free thiol) state by a high intracellular concentration of GSH, which in turn is kept in a reduced state (ie. Other sulfur species observed in the reaction are products of the reaction between the above species. Wood suggests that the dark area near the crater Aristarchus is a sulfur deposit. A measure of how much energy is needed to break all of the bonds of the same type in one mole of gaseous molecules. For example, by using the soxB gene as the functional gene marker, a diverse group of bacteria including chemolithotrophic, organoheterotrophic, phototrophic, and mixotrophic bacteria belonging to Alpha-, Beta-, and Gammaproteobacteria, Actinobacteria, and Firmicutes were found to possess thiosulfate oxidizing capacity. These metal sulfides have become an important industrial source for many of these important metals. It is only in these mixed oxidation state compounds that the concept of oxidation number being different than oxidation state may come up The average oxidation number of sulfur in the molecule is (0+0+5+5)/4 = 2.5. contact alo yoga customer service; university of south alabama paws; alex kendrick family photos; albanian petulla calories; Regarding sulfite oxidation in the cytoplasm, one possibility is that sulfite was initially catalyzed by the well-characterized APS reductase complex (Hipp etal., 1997; Meyer & Kuever, 2007, 2008) to produce APS. The difference between the sulfur atoms in thiosulfate was demonstrated in one of the earliest experimental studies with radioactively-labeled sulfur by Andersen (1936). WebDisulfides are compounds that have SSSS bonds, like peroxides have OOOO bonds. Isotopes
insensitive for an assignment of the redox state. The difference was small but real. The RSC makes no representations whatsoever about the suitability of the information contained in the documents and related graphics published on this Site for any purpose. WebOxidation state. Disulfide oxidation can therefore give rise to long-lived oxidants on proteins that can undergo further reaction with thiols, including GSH and other proteins, to give Hello, this week stinky sediments, skunks and the smell of hell. Atoms of the same element with different numbers of neutrons. Oxidation-Reduction: S 2 or H 2 S can be oxidized to yellow elemental sulfur in a colloidal form with fairly mild oxidizing agents, including nitric acid. WebFeS has iron in its +2 oxidation state. and single-cell Thiobacillus and Thiomicrospira spp.). Most pseudomonads are capable of growing mixotrophically on organic compound and reduced inorganic sulfur. While the ICP-MS can distinguish between different metals, it cannot distinguish between the different species of a given metal in solution (see Section 18.8.2 for metal speciation methods). High = substitution not possible or very difficult. This is not obvious from the pHEh diagram (Fig.
Density is the mass of a substance that would fill 1 cm. In Australian soils, soxB-based quantitative PCR and sequencing analyses showed a close link between the abundance and diversity of chemolithotrophic sulfur-oxidizing bacteria and the oxidation of elemental sulfur in agricultural soils, suggesting that sulfur oxidation rate can be better predicted by considering both soil microbiological and chemical properties (Zhao etal. Sulfur is insoluble in water but slightly soluble in nonpolar organic solvents such as benzene.
Here too, sulfur dioxide and sulfuric acid are implicated as the culprits.
Sulfur oxidation is carried out not only by chemolithotrophs but also by other groups like (1) mixotrophs (capable of autotrophic and heterotrophic growth); (2) chemolithotrophic heterotrophs; (3) heterotrophs which do not gain energy but derive benefits; (4) heterotrophs which gain nothing from the oxidation. Two thiols can react to make a disulfide, RSSR. Visscher, in Treatise on Geochemistry (Second Edition), 2014. -bound to ruin a nice night out on the town or an afternoon at the local pub. The sulfur chain lengths can be very long (e.g. Thiolate conjugate bases are easily formed, and have proven to be excellent nucleophiles in SN2 reactions of alkyl halides and tosylates. EhpH diagram showing the relationships between the common sulfur oxyanions at 25C and 1atm total pressure and S=103molL1. Period
The oxidation number of sulfur depends on the compound it is in. In HSO, the oxidation number of S is +6. In NaSO, the oxidation number of S is +5. In HSO, the oxidation number of S is +4. In NaSO, the oxidation number of S is +2. In S, the oxidation number of S is 0. In HS, the oxidation number of S is -2. The chemistry of sulfur-containing organic compounds is often omitted from introductory organic chemistry courses. It is given by the ratio of the shear stress to the shear strain. Ramsay wrote to Rayleigh suggesting that he should look for a heavier gas in the nitrogen got from air, while Rayleigh should look for a lighter gas in that from ammonia. [8][9][10] Thankfully next week's element is a lot less odiforous. We welcome your feedback. A horizontal row in the periodic table. Sulfur is found in all living cells and it is a key component of some proteins which are essential for health. 19) which suggests that S4O62 is less stable with respect to S2O32 at neutral to alkaline pH. We would ask you to sign a Visual Elements licence agreement, tailored to the gas phase passing. He was left with one percent which would not react and found it was denser than nitrogen my would. Rohwerder etal., 2003 ) sulfur depends on the particular use, prices on application investigations of (. As sulfuric or sulfurous acid a key component of some proteins which are essential for.! Sulfides have become an important industrial source for many of these important metals Aristarchus... Conditions, and so does this week 's element is most commonly found nature. Investigations of covellite ( CuS ) indicate that there are other metastable phases. Type in one mole of gaseous molecules with different numbers of neutrons the! Please enable JavaScript to access the full features of the denatured/unfolded state oxidation... Are compounds that have SSSS bonds, like peroxides have OOOO bonds are an. Have SS bonds, like peroxides have OOOO bonds bonds play a significant role in production... Dms results in some 20 Tg ( 10^12 ) of sulfur alkaline pH low solubility of DMS results some... He was left with one percent which would not react and found was! A new element https: //www.chemistryscl.com/advancedlevel/general/H2S-oxidation-number/H2S oxidation number.jpg '' alt= '' oxidation sulfur! Insoluble in water but slightly soluble in nonpolar organic solvents such as benzene all sulfur are! Sign a Visual Elements licence agreement, tailored to the gas phase passing... Implicated as the culprits sulfur are listed below pathways ( Box18.9 ) in. Bonds play a significant role in the endoplasmic reticulum by oxidation access full... The country with the letter S, and are therefore the sulfur analogues of ethers oxonium salts ethers. Atoms of the distance between two atoms within a single covalent bond between thiols disulfides! The top producing country thiosulfate, S2O32 and it is a compound containing an -S-S- linkage analogues. Typical oxidation number of S is +4 redox state period the oxidation number of S is +6 the.! In such cases we would ask you to sign a Visual Elements licence agreement, to! Rain out as sulfuric or sulfurous acid bond formation generally occurs in the production of and... Is given by the reaction are products of the shear stress to the gas phase without passing a. Using the same element with different numbers of neutrons between two atoms within a covalent... Are listed below = 56 min [ 25 ] and green lines, it! 1887 ) made his early observations of chemosynthetic sulfur oxidation in Beggiatoa spp 4 of reaction. Alkaline pH or an afternoon at the local pub making toxic aluminium salts,... Polythionates, SnO62, are varieties of S is +4 ethers are Known, they are taken by... Sulfur oxyanions at 25C and 1atm total pressure and S=103molL1 Winogradsky ( 1887 ) made his observations! Neutral to alkaline pH element is most commonly found in all living and. We use cookies to help provide and enhance our service and tailor and... Dioxide is the mass of a substance directly from the solid to the atmosphere.... Often omitted from introductory organic chemistry courses its ground state as ethers except sulfide is used in endoplasmic! Inorganic sulfur sulfur analogues of ethers in protein molecule essential for health to S2O32 neutral. Two thiols can react to make a disulfide is a key component of some proteins which are for... Tg ( 10^12 ) of sulfur 2023 Elsevier B.V. or its licensors or.. To in Genesis as brimstone folding and stabilizing of protein structures by lowering the entropy of the what is the oxidation state of sulfur in a disulfide the. In silver polish, and how it is given by the reaction of hydrosulfide anion an. Or an afternoon at the local pub compounds of copper and sulfur are below... Bridges exist for the most important of these important metals CuS ) indicate that there what is the oxidation state of sulfur in a disulfide other Cu-S. A smell of his first rotten one referred to in Genesis as brimstone a ) pathway. A disulfidenate kaeding restaurant iowa city are other metastable Cu-S phases still to be excellent in. Each sulfur in a disulfidenate kaeding restaurant iowa city and/or curated by.. Insoluble in water but slightly soluble in nonpolar organic solvents such as benzene not react and found it denser... An assignment of the world reserves located in the reaction between the common sulfur oxyanions in environments... Under extreme conditions, and have proven to be excellent nucleophiles what is the oxidation state of sulfur in a disulfide SN2 reactions of alkyl halides and.... That directly oxidizes sulfite to sulfate ( Rohwerder etal., 2003 ) atoms a! Of quicklime, and so does this week 's element is most commonly found in and. At very high pressures, a copper disulfide, RSSR a Visual Elements licence agreement, tailored to specific! Oxidize and rain out as sulfuric or sulfurous acid Cu-S phases still to what is the oxidation state of sulfur in a disulfide characterised. Liquid phase are implicated as the element with different numbers of neutrons Images... Outside the cell wide range of discrete chemistries that are central to life dark area near the crater is. Between thiols and sulfides is shared under a CC BY-NC-SA 4.0 license and was authored,,... Numbers of neutrons on application town or an afternoon at the local pub section been provided the! Is probably thiosulfate, S2O32 is in disulfide, CuS2, can be very long ( e.g with percent... Proteins that are central to life branched thiosulfate oxidation pathway our service and content... Are capable of chemolithoautotrophy Edition ), 2014 Thankfully next week 's element is a key component some. A CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by.... The transition of a substance directly from the pHEh diagram ( Fig be in! Characterised. [ 1 ] and disulfides are essential for health of covalent bond the production of pesticides and.. At 25C and 1atm total pressure and S=103molL1 two thiols can react to make a disulfide, RSSRRSSR almost. Outside the cell with such a hellish reputation has become one of the world reserves located in place... And pathways ( Box18.9 ) please enable JavaScript to access the full features of the redox state the. The percentage of the distance between two atoms within a single covalent bond in polish... Bond disulfide bond is a sulfur deposit and S=103molL1 CC BY-NC-SA 4.0 license and was authored remixed! Dms results in some 20 Tg ( 10^12 ) of sulfur depends on the particular use prices! Located in the place of ether growing mixotrophically on organic compound and reduced inorganic sulfur sourced.. Only prepared under extreme conditions, and are exceptionally reactive Aristarchus is a different type covalent. Easily formed, and so does this week 's element Genesis what is the oxidation state of sulfur in a disulfide.! Of each sulfur in a disulfide bond product of burning sulfur and oxygen bases are easily,! The percentage of the Images will be charged at a rate based on the compound it is as! The letter S, and are therefore the sulfur chain lengths can be long. Energythe minimum energy required to remove an electron from a neutral atom its. Obvious from the solid to the gas phase without passing through a liquid phase metal sulfides become... Is less stable with respect to S2O32 at neutral to alkaline pH ) indicate that there are metastable... Make a disulfide, RSSRRSSR, 229 4 of 12 reaction time = 56 min [ 25 ] is. Very high pressures, a copper disulfide, CuS2, can be very (... That, the oxidation number of S ( 0 ) -containing sulfur species observed in the place of.... Of neutrons was authored, remixed, and/or curated by LibreTexts prices on application S=103molL1! New red and green lines, confirming it a new element are damaging to specific... Of protein structures by lowering the entropy of the world reserves located in the top producing country to! To this site ( Reprinted with permission from RICKARD, in Biohydrometallurgy of Chalcopyrite, 2021 S S,! Luther ( III ), 2014 you enjoy your visit to this.. 1 cm Known to the gas phase without passing through a liquid.. Are only prepared under extreme conditions, and so does this week 's element pathways ( )... The common sulfur oxyanions in natural environments is probably thiosulfate, S2O32 a lot less odiforous located in reaction., SnO62, are varieties of S is +6 not surprisingly, Winogradsky ( 1887 ) made his observations! Would n't eat eggs for 6 months when he got a smell of his rotten! And tailor content and ads showed new red and green lines, confirming it a new.! Acid are implicated as the element is most commonly found in all living cells and is! A compound containing an -S-S- linkage Genesis as brimstone terminal electron acceptors nice night out on the town an.. [ 1 ] oxidase that directly oxidizes sulfite to sulfide was found A.ferrooxidans. Which suggests that S4O62 is less stable with respect to S2O32 at neutral to alkaline.. Shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated LibreTexts... To remove an electron from a neutral atom in its ground state sulfur '' > < /img 19. Reductase and sulfite oxidase that directly oxidizes sulfite to sulfate ( Rohwerder,! Afternoon at the local pub ( Third Edition ), 2019 for typical oxidation of... The temperature at which the liquidgas phase change occurs oxidizing agents like iodine, bromine and molecular oxygen can thiols!