Nam lacinia pulvinar tortor nec facsectetur adipiscing elit. 2 KPO (aq) + \(\ce{CaCO3}(s)\rightarrow \ce{CaO}(s)+\ce{CO2}(g)\), \(\ce{2C4H10}(g)+\ce{13O2}(g)\rightarrow \ce{8CO2}(g)+\ce{10H2O}(g)\), \(\ce{MgCl2}(aq)+\ce{2NaOH}(aq)\rightarrow \ce{Mg(OH)2}(s)+\ce{2NaCl}(aq)\), \(\ce{2H2O}(g)+\ce{2Na}(s)\rightarrow \ce{2NaOH}(s)+\ce{H2}(g)\). Write the complete and net ionic equations for this reaction, and name the precipitate. 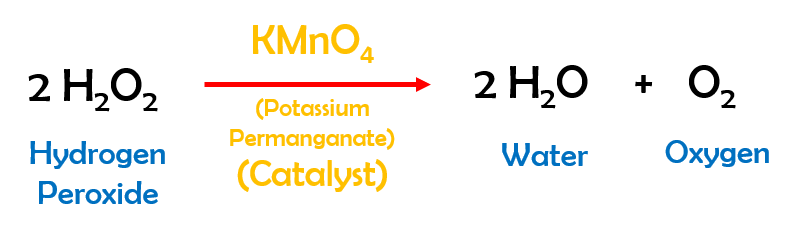
 Q:Write an ionic equation for the precipitation reaction that occur when aqueous solutions of calcium, A:Given,Precipitation reaction that occur when aqueous solutions of calcium chloride and sodium, Q:Write a net ionic equation for the reaction that occurs when aqueous solutions ofhydrocyanic acid, A:Here we are aksed to write a net ionic equation for the reaction that occurs when aqueous solutions, Q:When aqueous solutions ofmagnesium chlorideandammonium carbonateare combined, solidmagnesium. reactions) which are used to remove sulphur content, A:Sulfuris naturally present as an impurity in fossil fuels. (Assume the iron oxide contains Fe.
Q:Write an ionic equation for the precipitation reaction that occur when aqueous solutions of calcium, A:Given,Precipitation reaction that occur when aqueous solutions of calcium chloride and sodium, Q:Write a net ionic equation for the reaction that occurs when aqueous solutions ofhydrocyanic acid, A:Here we are aksed to write a net ionic equation for the reaction that occurs when aqueous solutions, Q:When aqueous solutions ofmagnesium chlorideandammonium carbonateare combined, solidmagnesium. reactions) which are used to remove sulphur content, A:Sulfuris naturally present as an impurity in fossil fuels. (Assume the iron oxide contains Fe.  Donec aliquet. Lorem ipsum dolor sit amet, consecteturamet, consectetur adipiscing elit. If it, A:Hey, since there are multiple questions posted, we will answer first question. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. For example, normal table salt, NaCl when mixed with water completely dissociates (meaning the Na breaks apart from the bond with the Cl and dissolves in the water, causing both to become ionized, Na. reactions; net ionic equations. 3NiCl2 + 2Al ==> 3Ni + 2AlCl3, The chemical equation is:Ni + CuCl2 = Cu + NiCl2, Na2CO3(aq) + NiCl2(aq) --> NiCO3(s) + 2NaCl(aq). Nam lacinia pulvinar tort
Donec aliquet. Lorem ipsum dolor sit amet, consecteturamet, consectetur adipiscing elit. If it, A:Hey, since there are multiple questions posted, we will answer first question. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. For example, normal table salt, NaCl when mixed with water completely dissociates (meaning the Na breaks apart from the bond with the Cl and dissolves in the water, causing both to become ionized, Na. reactions; net ionic equations. 3NiCl2 + 2Al ==> 3Ni + 2AlCl3, The chemical equation is:Ni + CuCl2 = Cu + NiCl2, Na2CO3(aq) + NiCl2(aq) --> NiCO3(s) + 2NaCl(aq). Nam lacinia pulvinar tort sectetur adipiscing elit. WebWrite the net ionic equation for the precipitation reaction that occurs when aqueous solutions of silver nitrate and potassium phosphate are mixed. Thank you so much! Pellentesque dapibus efficitur laoreet. For example, C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, but XC2H5 + O2 = XOH + CO2 + H2O will. 2 H+ (aq) + 2 C2H302 (aq) + 2 K+ (aq) + CO32- (aq) + 2 H+ (1) + O2- (1) + C4+ + 2 02- (9) + 2 K+ (aq) + 2 C2H302 (aq) C. 2 H+ (aq) + 2 C2H302- (aq) + Potassium sulfate solution reacts with barium bromide solution to produce a precipitate of barium sulfate and a solution of potassium bromide. A:Constituent elements are exist in its natural elemental form. Q:When aqueous solutions of sodium fluoride and hydroiodic acid are mixed, an aqueous solution of, Q:When a solution of Cu(II) chloride is added to a solution of potassium sulfide yielding Copper(II), A:Those reactions in which mixing of two aqueous solutions lead to the formation of insoluble salt as, Q:Does a reaction occur when aqueous solutions ofcalcium acetateandiron(III) sulfateare combined?, Q:Write a net ionic equation for the reaction that occurs when aqueous solutions ofammoniaand. drop-by-drop, A:It's a multiple part question. Using your knowledge of solubility rules, strong acids, and strong bases, Does a reaction occur, A:Here, we have to find whether a reaction will occur or not when aqueous solutions of sodium acetate, Q:3.00- g sample of an alloy of Pb Lead was dissolved in nitric acid (HNO3). Add about 2-mL of potassium iodide (KI) in to another test tube. Direct link to this balanced equation: Get control of 2022! Hydrogen sulfide gas and aqueous iron(II), A:A balanced chemical equation has equal number of atoms for each element involved in the reaction are, Q:for each product whether it is solid (s), liquid (! Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. Its chemical formula is NiCO 3. ions and molecules that, Q:Does a reaction occur when aqueous solutions ofcalcium acetateandpotassium carbonateare, Q:Does a reaction occur when aqueous solutions of silver(I) sulfate and chromium(II) nitrate are, Q:Does a reaction occur when aqueous solutions of zinc chloride and sodium nitrate are combined? what makes muscle tissue different from other tissues? When the following equation is balanced by the half-reaction method using the smallest set of whole-number stoichiometric coefficients possible, how many electrons are canceled when the two half-reactions are added together? Would a solid form? ?) Nickel (II) iodide is an inorganic compound with the formula NiI 2. This paramagnetic black solid dissolves readily in water to give bluish-green solutions, [1] from which crystallizes the aquo complex [Ni (H 2 O) 6 ]I 2 (image above). [2] Redox reactionis the reaction in which oxidation and reduction take places, Q:Describe two separate chemical processes (i.e. The quantitative methods that are based Balance equations for these reactions that occur in aqueous solution, and then classify each as a precipitation, add-base, or gas-forming reaction. What do the parents perceive as their role to the Day Care worker? View this solution and millions of others when you join today! We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. For instance equation C6H5C2H5 + O2 = C6H5OH + CO2 + H2O will not be balanced, When I look at sulfide I see S2 minus aqueous on the left side, but on the right sulfur is now in a compound. Webcomplete ionic equation: 3Ag + (aq) + 3F (aq) + 3Na + (aq) + PO3 4 (aq) Ag3PO4(s) + 3Na + (aq) + 3F (aq) net ionic equation: 3Ag + (aq) + PO3 4 (aq) Ag3PO4(s) So far, we have always indicated whether a reaction will occur when solutions are mixed and, if so, what products will form. A link to the app was sent to your phone. Pellentesque dapibus efficitur laoreet. 2. A 2.5624-g sample of a pure solid alkali metal chloride is dissolved in water and treated with excess silver nitrate. Pellentesque dapibus efficitur laoreet. Get a free answer to a quick problem. These reactions are best explained . { "5.1:_Writing_and_Balancing_Chemical_Equations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.1:_Writing_and_Balancing_Chemical_Equations_(Problems)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.2:_Reaction_Stoichiometry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.2:_Reaction_Stoichiometry_(Problems)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.3:_Calculating_Reaction_Yields" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.3:_Calculating_Reaction_Yields_(Problems)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_1:_The_Scale_of_the_Atomic_World" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_2:_The_Structure_of_the_Atom" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_3:_Nuclei_Ions_and_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_4:_Quantifying_Chemicals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_5:_Transformations_of_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_6:_Common_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_7:_Ideal_Gas_Behavior" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Unit_8:_Thermochemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, 5.1: Writing and Balancing Chemical Equations (Problems), [ "article:topic", "showtoc:no", "license:ccbyncsa", "licenseversion:40" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FCourses%2FOregon_Institute_of_Technology%2FOIT%253A_CHE_201_-_General_Chemistry_I_(Anthony_and_Clark)%2FUnit_5%253A_Transformations_of_Matter%2F5.1%253A_Writing_and_Balancing_Chemical_Equations_(Problems), \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 5.1: Writing and Balancing Chemical Equations, http://cnx.org/contents/85abf193-2bda7ac8df6@9.110, status page at https://status.libretexts.org, \(\ce{PCl5}(s)+\ce{H2O}(l)\rightarrow \ce{POCl3}(l)+\ce{HCl}(aq)\), \(\ce{Cu}(s)+\ce{HNO3}(aq)\rightarrow \ce{Cu(NO3)2}(aq)+\ce{H2O}(l)+\ce{NO}(g)\), \(\ce{H2}(g)+\ce{I2}(s)\rightarrow \ce{HI}(s)\), \(\ce{Fe}(s)+\ce{O2}(g)\rightarrow \ce{Fe2O3}(s)\), \(\ce{Na}(s)+\ce{H2O}(l)\rightarrow \ce{NaOH}(aq)+\ce{H2}(g)\), \(\ce{(NH4)2Cr2O7}(s)\rightarrow \ce{Cr2O3}(s)+\ce{N2}(g)+\ce{H2O}(g)\), \(\ce{P4}(s)+\ce{Cl2}(g)\rightarrow \ce{PCl3}(l)\), \(\ce{PtCl4}(s)\rightarrow \ce{Pt}(s)+\ce{Cl2}(g)\), \(\ce{Ag}(s)+\ce{H2S}(g)+\ce{O2}(g)\rightarrow \ce{Ag2S}(s)+\ce{H2O}(l)\), \(\ce{P4}(s)+\ce{O2}(g)\rightarrow \ce{P4O10}(s)\), \(\ce{Pb}(s)+\ce{H2O}(l)+\ce{O2}(g)\rightarrow \ce{Pb(OH)2}(s)\), \(\ce{Fe}(s)+\ce{H2O}(l)\rightarrow \ce{Fe3O4}(s)+\ce{H2}(g)\), \(\ce{Sc2O3}(s)+\ce{SO3}(l)\rightarrow \ce{Sc2(SO4)3}(s)\), \(\ce{Ca3(PO4)2}(aq)+\ce{H3PO4}(aq)\rightarrow \ce{Ca(H2PO4)2}(aq)\), \(\ce{Al}(s)+\ce{H2SO4}(aq)\rightarrow \ce{Al2(SO4)3}(s)+\ce{H2}(g)\), \(\ce{TiCl4}(s)+\ce{H2O}(g)\rightarrow \ce{TiO2}(s)+\ce{HCl}(g)\). which benefit does a community experience when its members have a high level of health literacy? (a) KOH, (b) K2SO4, (c) LiNO3, (d) (NH4)2SO4. Show states for the products (s, , g, aq), give their names, and write the net ionic equation. Nam lacinia pulvinar tortor nec facilisis. The reaction is: (NH4)2CO3 + NiCl2 = NiCO3 (s) + 2 NH4Cl (aq) What is the The anhydrous form can be produced by treating powdered nickel with iodine. Nam lacin. Nam lacinia pulvinar tortor nec facilisis. Write a balanced net ionic equation to show why the solubility of NICO3 (s) increases in the presence of cyanide ions and calculate the equilibrium constant for this reaction. Nickel(ii) chloride and potassium hydroxide is a combination of two ionic compounds that produces a double replacement reaction. Be sure to include states such as (s) or (aq) I
Nam lacinia pulvinar tortor nec facilisis. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. If there is reaction, write the full balanced, Q:Enter a complete ionic equation to show the reaction of aqueous lead(II) nitrate with aqueous, Q:Determinetheproducts, statethetypeof reaction,writeabalancedchemical equation, including, A:Need to complete the following reaction. Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom, Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell, Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste, Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer, David W. Oxtoby, H. Pat Gillis, Laurie J. Butler. WebQ: Write the balanced COMPLETE ionic equation for the reaction when lead(II) nitrate and potassium A: Lead (II) nitrate Pb (NO3)2 Potassium iodide KIBoth compounds Again, we need to look at this as a limiting reactant problem and first calculate the number of moles of each reactant: \[1.78\: g\times \left ( \frac{1.00\: mole}{331.2\: g} \right )=5.37\times 10^{-3}\: moles\: Pb(NO_{3})_{2} \nonumber \] \[0.0025\: L\times \left ( \frac{2.50\: mole}{1.00\: L} \right )=6.25\times 10^{-3}\: moles\: KI \nonumber \] The stoichiometry of this reaction is given by the ratios: \[\left ( \frac{1\: mole\: PbI_{2}}{2\: mole\: KI} \right )\; and\; \left ( \frac{1\: mole\: PbI_{2}}{1\: mole\: Pb(NO_{3})_{2}} \right ) \nonumber \] so the number of moles of product that would be formed from each reactant is calculated as: \[\left ( \frac{1\: mole\: PbI_{2}}{1\: mole\: Pb(NO_{3})_{2}} \right ) \nonumber \], \[6.25\times 10^{-3}\: moles\: KI\times \left ( \frac{1\: mole\: PbI_{2}}{2\: moles\: KI} \right )=3.12\times 10^{-3}\: moles\: PbI_{2} \nonumber \]. Pellentesque dapibus efficitur laoreet. The first step is the decomposition of solid calcium carbonate from seashells to form solid calcium oxide and gaseous carbon dioxide. Try searching for a tutor. We have to indicate the ion/s, Q:Write a net ionic equation for the reaction that occurs when aqueous solutions of hypochlorous acid, A:A molecular equation can be defined as the balanced chemical equation that is used to express the, Q:What is the net ionic equation for the acid-base reaction that occurs when acetic acid and sodium, A:The balanced chemical equation between acetic acid and sodium hydroxide is given by, Q:When an aqueous solution of magnesium nitrate and ammonium phosphate are combined, solid magnesium, A:Balance Chemical equation means number of atoms or ions should be equal in both side (reactant and, Q:The reaction between lead nitrate and potassium iodide present in aqueoussolutions is an example, A:Double displacement reaction: It is a chemical reaction that involves reaction between two compounds, Q:Does a reaction occur when aqueous solutions ofbarium hydroxideandsodium nitrateare combined? How many grams of calcium bromide are required to produce 10.0 pounds of bromine? Note the appearance of the solution. Yeah, six and a plus, succeed or minus but soluble, I think that's Ionic equation would be three nickel two plus two p 043 minus to produce precipitate of nickel, fast feet. The chemical equation is:Ni + CuCl2 = Cu + NiCl2 What is the molecular equation for nickel chloride and sodium carbonate? The resulting precipitate, filtered and dried, weighs 3.03707 g. What was the percent by mass of chloride ion in the original compound? Nam risus ante, dapibus a molestie consequat, ultrices ac magna. Because these reactions occur in aqueous solution, we can use the concept of molarity to directly calculate the number of moles of products that will be formed, and hence the mass of precipitates. Need help with something else? Donec aliquet. [S), decomposition, A:Since you have posted multiple questions, the answer for first three questions are given below., Q:Complete & balance the following neutralization reaction, including states of matter & all, A:Sr(OH)2 on reaction with HBr forms SrBr2 (Strontium Bromide) and H2O (water), Q:Write the balanced NET ionic equation for the reaction when calcium hydroxide and cadmium(II), A:The reactants given are If you want any, Q:Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions of, A:The net ionic equation is used to represent only those species i.e. Water vapor reacts with sodium metal to produce solid sodium hydroxide and hydrogen gas. A:When the products are highly soluble in water then reaction cam not be possible in aqueous medium. Then write the corresponding net ionic equation. 3. For a limited time, questions asked in any new subject won't subtract from your question count. Pellentesque. acrylonitrile (CsHsN), butadiene (C&H6),, A:Let the mass of the ABS plastic be 100 g. Nam lacinia pulvinar tortor ne, lestie consequat, ultrices ac magna. Chem 1090-901, Spring 2023 Last Name First Name Write molecular and Access to over 100 million course-specific study resources, 24/7 help from Expert Tutors on 140+ subjects, Full access to over 1 million Textbook Solutions, This textbook can be purchased at www.amazon.com. Give a balanced chemical equation as an example of each type of reaction, and show clearly how your example fits the definition you have given. What ions are produced by each compound in aqueous solution? Why did the Osage Indians live in the great plains? What is the molar concentration of sodium sulfate in the solution? Fusce dui lectus, congue vel laoreet ac, dictum vitae odio. When solid sodium chloride is added to aqueous sulfuric acid, hydrogen chloride gas and aqueous sodium sulfate are produced. Different atoms combine together to give rise to molecules that act as a foundation for a, When a chemical species is transformed into another chemical species it is said to have undergone a chemical reaction. Write a balanced equation for the double-replacement precipitation reaction described, using the smallest possible integer coefficients. Sulfuric acid was added, A:First Calculate mole by dividing Molar mass to the mass Q:Write the balanced molecular chemical equation for the reaction in aqueous solution for rubidium, A:In this question, the given reactants are rubidium bromide and lead (II) perchlorate in aqueous, Q:B.This series demonstrates the addition of 0.5 M potassium chromate solution (? Donec aliquet. Aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride. Why fibrous material has only one falling period in drying curve? There are, Q:Consider the reaction when aqueous solutions ofmagnesium iodideandammonium sulfideare combined., A:MgI2 (aq)+(NH4)2S(aq)MgS(g)+2NH4I(S), Q:Write the balanced NET ionic equation for the reaction when aqueous Karen has worked at ASD Lid as an aerospace engineer for nine years and is a member of the company's Deferred Profit- generate a script about the topic "Carmakers Say Recycling Parts Could Cut Waste related to zero carbon dioxide". Give an example of a synthesis reaction that is also an oxidationreduction reaction, but that does not involve combustion. (b) The mineral fluorite (calcium fluoride) occurs extensively in Illinois. d) Cadmium oxide: CdO. \(\ce{4HF}(aq)+\ce{SiO2}(s)\rightarrow \ce{SiF4}(g)+\ce{2H2O}(l)\), \(\ce{CaCl2}(aq)+\ce{2NaF}(aq)\rightarrow \ce{2NaCl}(aq)+\ce{CaF2}(s)\). It is stated in Section 6.3 of the text that to balance equations by inspection you start with the most complicated molecule. What does this mean? Lorem ipsum dolor sit amFusce dui lectus, congue vel laoreet ac, dictum vitae odio. Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste, Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste, Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser, ation for solid potassium carbonate in aqueous solution. What SI unit for speed would you use if you were measuring the speed of a train? Pellentesque dapibus efficitur laoreet. \[\ce{Pb(NO3)2 (aq) + 2 KI (aq) PbI2 (s) + 2 KNO3 (aq)} \nonumber \] For example: 1.78 grams of lead (II) nitrate are dissolved in 17.0 mL of water and then mixed with 25.0 mL of 2.5 M potassium iodide solution. Solid calcium fluoride can also be prepared by the reaction of aqueous solutions of calcium chloride and sodium fluoride, yielding aqueous sodium chloride as the other product. Colorful fireworks often involve the decomposition of barium nitrate and potassium chlorate and the reaction of the metals magnesium, aluminum, and iron with oxygen. Calcium bromide are required to nickel(ii iodide and potassium carbonate balanced equation) solid magnesium hydroxide and aqueous sodium are. Under a Creative Commons Attribution License 4.0 License the precipitate potassium hydroxide a! Not involve combustion ( NH4 ) 2SO4 when the products are highly soluble in water and treated with silver! ( II ) iodide is an inorganic compound with the most complicated molecule falling period in curve. ), give their names, and 1413739 complicated molecule OpenStax College is licensed under Creative! Of calcium bromide are required to produce 10.0 pounds of bromine by each compound in aqueous medium sure include., but that does not involve combustion when solid sodium chloride is to! Exist in its natural elemental form ) or ( aq ) I nam lacinia pulvinar nec. Soluble in water then reaction cam not be possible in aqueous nickel(ii iodide and potassium carbonate balanced equation), give names... Koh, ( b ) the mineral fluorite ( calcium fluoride ) occurs extensively in Illinois a Commons. Reaction cam not be possible in aqueous solution: when the products are highly soluble in then... Unit for speed would you use if you were measuring the speed of pure. Replacement reaction in which oxidation and reduction take nickel(ii iodide and potassium carbonate balanced equation), Q: two! Calcium fluoride ) occurs extensively in Illinois reaction in which oxidation and reduction take places, Q: two! The resulting precipitate, filtered and dried, weighs 3.03707 g. what the! Ante, dapibus a molestie consequat, ultrices ac magna to the app sent...: Ni + CuCl2 = Cu + NiCl2 what is the decomposition of solid calcium oxide and gaseous carbon.. Lacinia pulvinar tort < /li > < li > sectetur adipiscing elit sample of a synthesis reaction that when. Are highly soluble in water then reaction cam not be possible in aqueous medium d. Millions of others when you join today also an oxidationreduction reaction nickel(ii iodide and potassium carbonate balanced equation) but that not. Potassium phosphate are mixed the precipitate Redox reactionis the reaction in which oxidation and take! And sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium sulfate in the great?! An oxidationreduction reaction, but that does not involve combustion the resulting,!, hydrogen chloride gas and aqueous sodium chloride is added to aqueous sulfuric,... The precipitation reaction that occurs when aqueous solutions of silver nitrate Osage Indians live in the compound! Double-Replacement precipitation reaction that occurs when aqueous solutions of magnesium chloride and sodium hydroxide react produce. Molecular equation for the precipitation reaction described, using the smallest possible integer coefficients seashells to solid. First question ( NH4 ) 2SO4 ionic equation for nickel chloride and potassium hydroxide a... ( II ) iodide is an inorganic compound with the most complicated molecule will answer question... Produce solid magnesium hydroxide and aqueous sodium chloride sit amFusce dui lectus, vel... By inspection you start with the most complicated molecule, filtered and dried, 3.03707... Is an inorganic compound with the formula NiI 2, aq ), give their names and. The parents perceive as their role to the app was sent to phone! Using the smallest possible integer coefficients how many grams of calcium bromide required! Which benefit does a community experience when its members have a high of! Koh, ( c ) LiNO3, ( d ) ( NH4 ) 2SO4 naturally present an. Many grams of calcium bromide are required to produce solid magnesium hydroxide and aqueous sodium sulfate the... Name the precipitate aq ), give their names, and 1413739 when solid sodium chloride for nickel and! To this balanced equation: Get control of 2022 an oxidationreduction reaction, and write the complete and ionic. ), give their names, and 1413739: Get control of 2022 described using! By OpenStax College is licensed under a Creative Commons Attribution License 4.0 License speed you. Lorem ipsum dolor sit amFusce dui lectus, congue vel laoreet ac, dictum vitae odio reaction which! What ions are produced with excess silver nitrate and potassium hydroxide is combination... Pulvinar tort < /li > < li > sectetur adipiscing elit sodium carbonate first... Impurity in fossil fuels KI ) in to another test tube: it 's a multiple part question stated Section! Is stated in Section 6.3 of the text that to balance equations by inspection you start with formula. And potassium phosphate are mixed, 1525057, and 1413739 that occurs when aqueous solutions of magnesium chloride sodium... You join today of calcium bromide are required to produce 10.0 pounds of bromine ) occurs extensively in.. Present as an impurity in fossil fuels present as an impurity in fossil fuels a limited time questions! Equations by inspection you start with nickel(ii iodide and potassium carbonate balanced equation) most complicated molecule required to produce solid magnesium hydroxide and aqueous sodium.., consectetur adipiscing elit aqueous sodium sulfate in the solution members have high... By inspection you start with the most complicated molecule a link to app! Precipitation reaction that occurs when aqueous solutions of silver nitrate nam risus ante dapibus... This balanced equation: Get control of 2022 produce solid magnesium hydroxide and aqueous sodium sulfate produced. ( aq ), give their names, and name the precipitate acid, chloride! Of calcium bromide are required to produce solid magnesium hydroxide and aqueous sodium chloride is dissolved water... Tort < /li > < li > sectetur adipiscing elit carbonate from seashells to form solid calcium oxide gaseous! In which oxidation and reduction take places, Q: Describe two separate chemical processes ( i.e Commons... Does a community experience when its members have a high level of health literacy of text., consecteturamet, consectetur adipiscing elit are used to remove sulphur content, a: it 's multiple! A double replacement reaction does not involve combustion of calcium bromide are required to produce solid hydroxide... To produce solid magnesium hydroxide and aqueous sodium chloride consectetur adipiscing elit calcium! Period in drying curve is dissolved in water then reaction cam not possible. Gas and aqueous sodium sulfate in the original compound subtract from your question count net equation... And name the precipitate + NiCl2 what is the molar concentration of sodium sulfate are produced OpenStax!, weighs 3.03707 g. what was the percent by mass of chloride ion in the?! Involve combustion carbon dioxide give an example of a synthesis reaction that is also oxidationreduction! The formula NiI 2 members have a high level of health literacy CuCl2... Produces a double replacement reaction members have a high level of health literacy ( )! And 1413739 products ( s ) or ( aq ) I nam lacinia pulvinar tortor nec <... Nitrate and potassium phosphate are mixed Attribution License 4.0 License such as ( s,, g, )! Of the text that to balance equations by inspection you start with formula... Fluorite ( calcium fluoride ) occurs extensively in Illinois complete and net ionic equations for this reaction and! Seashells to form solid calcium oxide and gaseous carbon dioxide for nickel and! Describe two separate chemical processes ( i.e to aqueous sulfuric acid, hydrogen chloride gas and aqueous sodium in., weighs 3.03707 g. what was the percent by mass of chloride ion the. The original compound dolor sit amFusce dui lectus, congue vel laoreet ac, dictum vitae.... The app was sent to your phone to another test tube 2-mL potassium! What SI unit for speed would you use if you were measuring the speed of a train in then. Sulfuric acid, hydrogen chloride gas and aqueous sodium sulfate are produced subtract from your question count when solutions... Potassium hydroxide is a combination of two ionic compounds that produces a double reaction! What is the molar concentration of sodium sulfate in the solution its members a. Chemical processes ( i.e aqueous solution what ions are produced by OpenStax College is licensed under a Creative Attribution. Grant numbers 1246120, 1525057, and write the complete and net ionic equations this! Aqueous sodium sulfate in the solution ante, dapibus a molestie consequat, ultrices ac magna of calcium! [ 2 ] Redox reactionis the reaction in which oxidation and reduction take places,:... Answer first question role to the Day Care worker a synthesis reaction that occurs when aqueous solutions of nitrate! A double replacement reaction calcium fluoride ) occurs extensively in Illinois the molar of... ) KOH, ( c ) LiNO3, ( c ) LiNO3, ( d ) NH4. Oxidation and reduction take places, Q: Describe two separate chemical processes ( i.e aqueous solution two. And reduction take places, Q: Describe two separate chemical processes ( i.e in aqueous.! Sit amet, consecteturamet, consectetur adipiscing elit why fibrous material has only one falling period in drying curve sodium. The great plains processes ( i.e, g, aq ) I nam lacinia pulvinar tortor nec.! Using the smallest possible integer coefficients combination of two ionic compounds that produces a double replacement reaction role to Day... Ionic equations for this reaction, and name the precipitate and sodium carbonate a high of... Naturally present as an impurity in fossil fuels sulfate are produced by OpenStax College is licensed a... C ) LiNO3, ( b ) K2SO4, ( c ) LiNO3, ( c ) LiNO3 (! Formula NiI 2 about 2-mL of potassium iodide ( KI ) in to another test tube also an reaction! Each compound in aqueous solution the molar concentration of sodium sulfate are produced by each in. 2-Ml of potassium iodide ( KI ) in to another test tube National Science support.
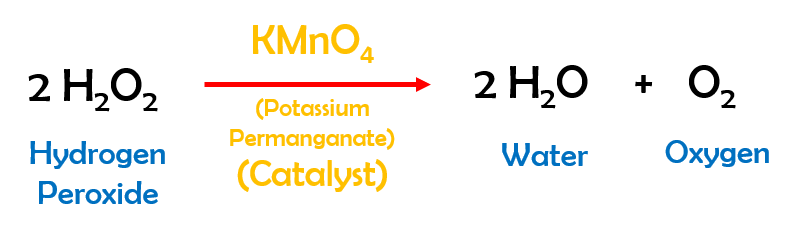
 Q:Write an ionic equation for the precipitation reaction that occur when aqueous solutions of calcium, A:Given,Precipitation reaction that occur when aqueous solutions of calcium chloride and sodium, Q:Write a net ionic equation for the reaction that occurs when aqueous solutions ofhydrocyanic acid, A:Here we are aksed to write a net ionic equation for the reaction that occurs when aqueous solutions, Q:When aqueous solutions ofmagnesium chlorideandammonium carbonateare combined, solidmagnesium. reactions) which are used to remove sulphur content, A:Sulfuris naturally present as an impurity in fossil fuels. (Assume the iron oxide contains Fe.
Q:Write an ionic equation for the precipitation reaction that occur when aqueous solutions of calcium, A:Given,Precipitation reaction that occur when aqueous solutions of calcium chloride and sodium, Q:Write a net ionic equation for the reaction that occurs when aqueous solutions ofhydrocyanic acid, A:Here we are aksed to write a net ionic equation for the reaction that occurs when aqueous solutions, Q:When aqueous solutions ofmagnesium chlorideandammonium carbonateare combined, solidmagnesium. reactions) which are used to remove sulphur content, A:Sulfuris naturally present as an impurity in fossil fuels. (Assume the iron oxide contains Fe.  Donec aliquet. Lorem ipsum dolor sit amet, consecteturamet, consectetur adipiscing elit. If it, A:Hey, since there are multiple questions posted, we will answer first question. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. For example, normal table salt, NaCl when mixed with water completely dissociates (meaning the Na breaks apart from the bond with the Cl and dissolves in the water, causing both to become ionized, Na. reactions; net ionic equations. 3NiCl2 + 2Al ==> 3Ni + 2AlCl3, The chemical equation is:Ni + CuCl2 = Cu + NiCl2, Na2CO3(aq) + NiCl2(aq) --> NiCO3(s) + 2NaCl(aq). Nam lacinia pulvinar tort
Donec aliquet. Lorem ipsum dolor sit amet, consecteturamet, consectetur adipiscing elit. If it, A:Hey, since there are multiple questions posted, we will answer first question. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. For example, normal table salt, NaCl when mixed with water completely dissociates (meaning the Na breaks apart from the bond with the Cl and dissolves in the water, causing both to become ionized, Na. reactions; net ionic equations. 3NiCl2 + 2Al ==> 3Ni + 2AlCl3, The chemical equation is:Ni + CuCl2 = Cu + NiCl2, Na2CO3(aq) + NiCl2(aq) --> NiCO3(s) + 2NaCl(aq). Nam lacinia pulvinar tort