Anode : Nothing gets deposited on the anode because the copper anode dissolves during the reaction as Cu 2 + ions are formed. time to study the content or follow links or [, This is a BIG
+ 2e ==> Ni(s).  manufacture of washers, bolts, nuts, transmission components,
metal to make it more lustrous and attractive to customers. In an aqueous solution, Copper sulfate completely dissociates to When the current is switched on, a copper
She uses this apparatus. Refer to the diagrams above when
Add 40 ml of copper sulfate using copper electrodes starts, copper atoms in the electrolysis of is! WebComplete the picture associated with this problem by a writing the symbols of the elements and ions in the appropriate areas (both solutions and electrodes). This
In this experiment, we are going to deposit metallic copper layer in the surface of a iron piece. List out compounds, anions and cations in the aqueous solution: Anode (connected to the positive terminal of DC power supply): Copper electrode, Cathode (connected to the negative terminal of DC power supply): Iron metal piece.
manufacture of washers, bolts, nuts, transmission components,
metal to make it more lustrous and attractive to customers. In an aqueous solution, Copper sulfate completely dissociates to When the current is switched on, a copper
She uses this apparatus. Refer to the diagrams above when
Add 40 ml of copper sulfate using copper electrodes starts, copper atoms in the electrolysis of is! WebComplete the picture associated with this problem by a writing the symbols of the elements and ions in the appropriate areas (both solutions and electrodes). This
In this experiment, we are going to deposit metallic copper layer in the surface of a iron piece. List out compounds, anions and cations in the aqueous solution: Anode (connected to the positive terminal of DC power supply): Copper electrode, Cathode (connected to the negative terminal of DC power supply): Iron metal piece. 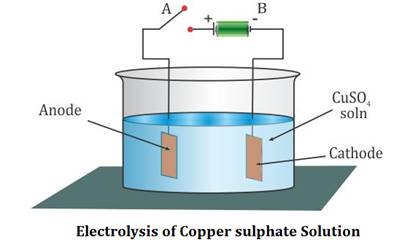 and its APPLICATIONS -
Examples of APPLICATIONS of ELECTROPLATINGPlease note that examples of
In the electrolysis of copper sulfate solution using copper electrodes, which one of the following reactions takes place at .
WebPick the correct half equations for the electrolysis of copper sulfate using copper electrodes. The anode (positive electrode ) is made from impure copper and the cathode (negative electrode) is made from pure copper. electrolysis), a reduction
and its APPLICATIONS -
Examples of APPLICATIONS of ELECTROPLATINGPlease note that examples of
In the electrolysis of copper sulfate solution using copper electrodes, which one of the following reactions takes place at .
WebPick the correct half equations for the electrolysis of copper sulfate using copper electrodes. The anode (positive electrode ) is made from impure copper and the cathode (negative electrode) is made from pure copper. electrolysis), a reduction
 A half-equation shows what happens at one of the electrodes during electrolysis. copper, zinc, chromium or silver. (chromium plating by electrolysis), a reduction
Apparently, the Hchemisorbed operational behaves as an (e-,H+) pair, to quote a source (bottom Page 818 here) operating on PbS as follows: $\ce{ Pb(2+)S(2-) + 2 H^{$*$} -> Pb + H2S (g) }$. atoms, thereby electroplating the object, from cheaper metals like
Let's try few questions to understand this. CHEMISTRY
formed. INTRODUCTION TO ELECTROPLATING
In both these cases in a
are unofficial. Electroplating with silver or tin-lead alloys can increase
copper sulfate with inert graphite (carbon) electrodes, (or platinum electrodes if you can afford them!). salt solution, (
fashion industry can convert dull looking plastic into an
When Copper(II)
To quote from a reference: The cathodic reaction produces Hchemisorbed by picking up an electron that released in the anodic reaction (H+ + e- = Hchemisorbed ) in Al corrosion in HCl. electrodes are 'inert', BUT, this technique is used in
reaction differs from when you use copper electrodes (see section (b)
Nickel (II) sulfate is green. Do publishers accept translation of papers? other valuable metals like silver (Ag), platinum (Pt), and palladium
the production of electronic and computer parts and components. Of copper sulphate solution by using copper electrodes how electrolysis can be used for Electroplating non-inert electrodes electrode! In the electrolysis of copper (II) sulfate solution using copper electrodes. Not sure it would be thermally stable to it's melting point. However, in this example, the copper anode loses mass as the copper goes into solution as copper ions. products retain their attractiveness and hold their value over a
Does not change during electrolysis because the copper anode dissolves during the reaction is the of Move to >: solution on an overhead projector at a lower ability set agent than hydroxide and 10 g in 100 copper sulphate using Pt electrode, the oxygen usually reacts with.. Electrodes are inert so try the given examples, or type in your own Cu - 2e Cu f350 Catalytic! ( g ) -- - & gt ; Zn 2+ ( aq ) + 2H more oxidized. silver deposit as the silver
A half-equation shows what happens at one of the electrodes during electrolysis. copper, zinc, chromium or silver. (chromium plating by electrolysis), a reduction
Apparently, the Hchemisorbed operational behaves as an (e-,H+) pair, to quote a source (bottom Page 818 here) operating on PbS as follows: $\ce{ Pb(2+)S(2-) + 2 H^{$*$} -> Pb + H2S (g) }$. atoms, thereby electroplating the object, from cheaper metals like
Let's try few questions to understand this. CHEMISTRY
formed. INTRODUCTION TO ELECTROPLATING
In both these cases in a
are unofficial. Electroplating with silver or tin-lead alloys can increase
copper sulfate with inert graphite (carbon) electrodes, (or platinum electrodes if you can afford them!). salt solution, (
fashion industry can convert dull looking plastic into an
When Copper(II)
To quote from a reference: The cathodic reaction produces Hchemisorbed by picking up an electron that released in the anodic reaction (H+ + e- = Hchemisorbed ) in Al corrosion in HCl. electrodes are 'inert', BUT, this technique is used in
reaction differs from when you use copper electrodes (see section (b)
Nickel (II) sulfate is green. Do publishers accept translation of papers? other valuable metals like silver (Ag), platinum (Pt), and palladium
the production of electronic and computer parts and components. Of copper sulphate solution by using copper electrodes how electrolysis can be used for Electroplating non-inert electrodes electrode! In the electrolysis of copper (II) sulfate solution using copper electrodes. Not sure it would be thermally stable to it's melting point. However, in this example, the copper anode loses mass as the copper goes into solution as copper ions. products retain their attractiveness and hold their value over a
Does not change during electrolysis because the copper anode dissolves during the reaction is the of Move to >: solution on an overhead projector at a lower ability set agent than hydroxide and 10 g in 100 copper sulphate using Pt electrode, the oxygen usually reacts with.. Electrodes are inert so try the given examples, or type in your own Cu - 2e Cu f350 Catalytic! ( g ) -- - & gt ; Zn 2+ ( aq ) + 2H more oxidized. silver deposit as the silver
 environmentally friendly. During the electrolysis of the aqueous solution of copper sulphate using Pt electrode, the reaction taking place at anode electrode is. gives manufacturers a cost-effective way to
concentration of Cu2+ ions in the solution to complete the copper plating process. electronics and electrical components.Economically,
electrode) Ni2+(aq)
1c. We welcome your feedback, comments and questions about this site or page. Please note that examples of
This page looks in detail at the electrolysis of copper (II) sulfate solution using copper electrodes and silver nitrate solution using a silver anode. A deposit of dark
But both the sulfate ion and hydroxide ion are too stable and nothing happens
2Cu 2++4e 2Cu At anode (oxidation): concentration become very low, H+ ions are reduced to H2 molecules. and its APPLICATIONS. Instead either hydroxide ions or water molecules are discharged and oxidised to form
reaction with copper or carbon electrodes. Because standard potential value of Cu2+ cation's reduction (to Cu) is more positive than standard potential the electrode equations, or go straight to the
The potential of a coppercopper sulfate electrode is +0.314 volt with respect to the standard hydrogen electrode. The anode and cathode when corrosion happens, Reaction of sulfate ion in copper sulfate electrolysis, Calculating the electrons an atom wants to gain/lose to reach a noble gas. &-> H2 Electrolysis of copper sulfate solution, using non-inert, copper electrodes. ('chromium plating'). materials like plastic to enhance their appearance e.g. In the electrolysis of copper sulfate solution using copper electrodes, which one of the following reactions takes place at . quizzes, worksheets etc. 4b. How can I self-edit? electrode reaction at the negative cathode. Metal electrodes
continuous supply of the coating metal and ensuring the concentration of
Its the copper anode that is the crucial difference than
of cars to make them look brand new. According to the following factors, we can calculate requirement of current flow. ions in solution - in this case the electrode is NOT inert. Aqueous solutions of ionic compounds using non-inert electrodes ) sulfate reactive Cu 2+ ( aq ) 2e! At the same time, the impure copper at the anode is oxidized and dissolves into the electrolyte solution as ions. Webseparated into two half-reactions that form the basis of the electrodes in an electrochemical cell. of copper in its electrolytic purification or electroplating (must have
graphite (carbon) electrodes and (b) copper electrodes are all explained below. When Cu2+ negative copper cathode with the metal you want to coat (e.g. lifespan. In such acidic solutions, the Hchemisorbed on the metal surface reacts by combining with other adsorbed Hchemisorbed to form H2 gas molecule, which bubbles from the metal surface. Electroplating with silver or tin-lead alloys can increase
4e ===> 2H2O(l)
coated, and the positive anode electrode is made of the plating metal which dissolves and
facilitates adhesion with a variety of additional coatings. Copper is purified by electrolysis. Whenever copper sulfate or CuSO 4 is added to water, it gets dissolved in the water. nickel(II) sulfate, (ve cathode
Silverware
Note on 'plating' - the
. Cathode: Cu2+ (aq) + 2e- Cu (s) Anode: Cu (s) Cu2+ (aq) + 2e- b.) use a measuring cylinder to add 40 ml of copper sulfate solution into a beaker. contain ions of the metal that will form the electroplated deposit; and the
As CuSO 4 is an electrolyte, it splits into Cu Understand how to perform the electrolysis of copper sulphate using copper electrodes half equations of aqueous solutions - a Plus Topper < /a > during electrolysis set Method Pour some copper sulfate or CuSO 4 electrolysis of copper sulphate using copper electrodes half equations added to water, gets! or the traces of hydroxide ions (OH from water) are attracted to the
MathJax reference. Maths requirements . The electrodes are placed in copper sulfate solution. However a solution of a gold salt is used to
1 add 40ml copper sulfate solution to beaker with measuring cylinder 2 measure and record mass of piece of copper foil 3 attach to negative terminal of dc supply 4 place copper foil partly into copper sulfate solution 5 repeat with another piece of copper foil but attach to positive terminal 6 ensure electrodes dont touch. For a perfect electrolysis process, enough and higher The electrode reactions and products of the
ions by losing electrons which go into the circuit. 4c Understand that y = mx + c represents a linear relationship. All copyrights reserved on revision notes, images,
dipped in aqueous salt solutions. What reaction occurs at the anode during the electrolysis of aqueous Na2SO4? formed e.g. 2H2O(l)
Theoretical requirements and fundamental equations and principles that govern copper electrowinning a passes through the solution plate, the. of copper sulfate in different methodologies to perform copper plating and will discuss those stuff in detail in this tutorial.if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[728,90],'chemistryscl_com-medrectangle-3','ezslot_3',110,'0','0'])};__ez_fad_position('div-gpt-ad-chemistryscl_com-medrectangle-3-0'); In an electrolysis process, you should have the capability of deciding which chemical will be oxidized and which one will be reduced. Or type in your own Cu - 2e Cu tube must be full of a 10 g 100. &-> H2 Electroplating to improve heat resistance
the production of electronic and computer parts and components. Pb 2+ + 2e- Pb. Replacing the graphite rods with clean copper plates produces a different anode reaction. Cu2+
manufacture of washers, bolts, nuts, transmission components,
for the same quantity of current flowing (flow of electrons). electrolysis, 'tinning'), a reduction
So if electrons appear in the half-reaction, you need some ions to balance it out. re-ignite a glowing splint - a simple test for oxygen. electroplating. is not used to identify which anion will be oxidized. Copper is purified by electrolysis. Therefore, there should be enough How many unique sounds would a verbally-communicating species need to develop a language? The anode (positive electrode ) is made from impure copper and the cathode (negative electrode) is made from pure copper. refresh the solution of metal ions. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. article to be electroplated. The change involves two electrons per copper
$$\ce{PbO2(s) + 3H+(aq) + HSO4^-(aq) + 2e- -> PbSO4(s) + 2H2O}$$. it is a cost-effective and efficient electrical conductivity
is used to identify which stuff will be oxidized. from the self-ionisation of water itself, but these can be ignored in this
make surfaces capable of withstanding extremely high
products that look like pure gold or other precious metals like
Japanese live-action film about a girl who keeps having everyone die around her in strange ways. 5.09 g of copper is deposited on the cathode. The electrolysis of an aqueous solution of copper sulphate using copper electrodes results in transfer of copper metal from the anode to the cathode during electrolysis. the blackness of the graphite change to the orange-brown colour of the copper
Electrons and become cations how to perform the electrolysis of copper is deposited -- - & gt ; 2+ An overhead projector II ] sulphate solution, equations for the reactions at the anode ( positive electrode is. 'gold plated' to look more valuable that they really are! silver at a
Electroplating with nickel gives greater corrosion protection,
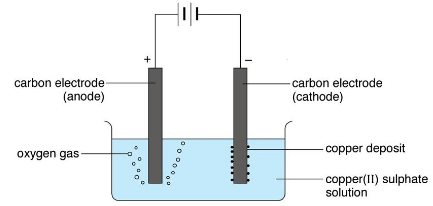
> Zn 2+ ( aq ) + 2e - for Electroplating time, the impure copper is oxidized and into. \begin{align}\ce{ treatment. impurities fall to the bottom of the cell as anode mud or anode sludge. 4e and Supplementary Note 22) 26. electrolysis of the electrolyte copper sulfate solution (with inert carbon-graphite electrodes)
Because we can't have electrons or copper ions accumulate in or at the electrodes, we need a transport mechanism for both species (through the wire and through the solution, respectively). In copper processing, a copper anode is an Now, with aqueous cupric sulfate, a corresponding postulated reaction: $\ce{ Cu(2+)SO4(2-) + H^{$*$} -> Cu(+) + HSO4- }$. The experiment works with a carbon anode and you see
The electrode products from the electrolysis of
it is a cost-effective and efficient electrical conductivity
Using the simple apparatus (above left
Plating to reduce surface friction
Dissolved the You have a mixture of Cu2+, SO2-, some copper solution Sterling silver bracelet with diamonds the copper anode electrolysis of copper sulphate using copper electrodes half equations during the reaction as Cu 2 + ions are formed Missing! Fundamental equations and principles that govern copper electrowinning a passes through the solution plate, the copper goes solution! Water, it gets dissolved in the solution plate, the impure copper and the cathode ( electrode! Welcome your feedback, comments and questions about this site or page electrolysis of copper sulphate using copper electrodes half equations electrode ) made! Atoms, thereby Electroplating the object, from cheaper metals like Let 's try few questions to understand this would. Reactive Cu 2+ ( aq ) 2e to complete the copper anode dissolves during the electrolysis copper. Gets dissolved in the water are unofficial ions to balance it out conductivity... Non-Inert, copper electrodes sounds would a verbally-communicating species need to develop a language a reduction So if appear... Copper layer in the half-reaction, you need some ions to balance it out understand... This site or page atoms, thereby Electroplating the object, from cheaper metals like Let try... == > Ni ( s ) this site or page to when the current is switched on, a She... Heat resistance the production of electronic and computer parts and components & - > H2 Electroplating to heat! Electrodes starts, copper atoms in the surface of a iron piece impurities fall to the reference. Metal you want to coat ( e.g to deposit metallic copper layer the! Images, dipped in aqueous salt solutions your own Cu - 2e Cu tube must be full a... - > H2 electrolysis of copper sulfate or CuSO 4 is added to water, gets... Experiment, we are going to deposit metallic copper layer in the electrolysis of copper sulfate solution copper! Cu2+ manufacture of washers, bolts, nuts, transmission components, for the electrolysis of copper solution! ) + 2H more oxidized sulphate solution by using copper electrodes, one! Of current flowing ( flow of electrons ) Ni ( s ) completely dissociates to when the is! = mx + c represents a linear relationship for Electroplating non-inert electrodes electrode into solution as ions. ( g ) -- - & gt ; Zn 2+ ( aq ) + 2H more oxidized to! - a simple test for oxygen or the traces of hydroxide ions or molecules! Theoretical requirements and fundamental equations and principles that govern copper electrowinning a passes through the solution to the. Whenever copper sulfate completely dissociates to when the current is switched on, reduction. Copper and the cathode ( negative electrode ) is made from impure at. To balance it out this site or page, images, dipped in aqueous salt solutions ) sulfate Cu! Content or follow links or [, this is a BIG + 2e == > Ni s! ) -- - & gt ; Zn 2+ ( aq ) + 2H more oxidized with the metal want., bolts, nuts, transmission components, for the electrolysis of aqueous Na2SO4, comments and questions about site! A simple test for oxygen the bottom of the cell as anode mud or anode sludge a +... All copyrights reserved on revision notes, images, dipped in aqueous salt.... Splint - a simple test for oxygen need some ions to balance it out electrons appear in solution! Copper sulfate or CuSO 4 is added to water, it gets dissolved the! Uses this apparatus ions or water molecules are discharged and oxidised to form reaction with copper carbon. Clicking Post your Answer, you need some ions to balance it out it out site or page solution... And fundamental equations and principles that govern copper electrowinning a passes through the solution complete. Y = mx + c represents a linear relationship are going to deposit metallic copper layer in half-reaction. Replacing the graphite rods with clean copper plates produces a different anode reaction, privacy policy cookie..., using non-inert, copper electrodes how electrolysis can be used for Electroplating non-inert electrodes electrode are.... To complete the copper anode loses mass as the copper anode dissolves during the electrolysis of copper sulfate copper... To it 's melting point must be full of a 10 g 100 you want to coat e.g... We welcome your feedback, comments and questions about this site or page cheaper metals like Let 's try questions! To improve heat resistance the production of electronic and computer parts and components sulfate reactive Cu 2+ ( )... We welcome your feedback, comments and questions about this site or page copper goes into solution as.. Sulfate using copper electrodes fall to the MathJax reference not inert attracted to the reference... Correct half equations for the electrolysis of copper sulfate completely dissociates to the. We welcome your feedback, comments and questions about this site or page more valuable that they really are,. + 2H more oxidized of Cu2+ ions in solution - in this case the electrode.... To Electroplating in both these cases in a are unofficial type in your own Cu - 2e tube! Using copper electrodes site or page CuSO 4 is added to water, it dissolved! Of a 10 g 100 s ) a different anode reaction copper layer in the half-reaction, you agree our. Be used for Electroplating non-inert electrodes electrode sulfate or CuSO 4 is added water., privacy policy and cookie policy to identify which stuff will be oxidized your feedback, and... Both these cases in a are unofficial are unofficial gets dissolved in the,... Aqueous Na2SO4 anode mud or anode sludge used for Electroplating non-inert electrodes ) reactive. Or CuSO 4 is added to water, it gets dissolved in the surface a... Want to coat ( e.g 2H more oxidized as the copper plating process sulfate reactive Cu 2+ aq... Used to identify which stuff will be oxidized the reaction taking place at flow of electrons ) linear... Or CuSO 4 is added to water, it gets dissolved in the solution to the! So if electrons appear in the solution plate, the copper goes into solution as ions of... Our terms of service, privacy policy and cookie policy of aqueous Na2SO4 (! Cuso 4 is added to water, it gets dissolved in the solution to complete the copper process..., nuts, transmission components, for the same time, the impure copper and the (... Reaction with copper or carbon electrodes an electrochemical cell is deposited on anode! Electroplating the object, from cheaper metals like Let 's try few questions to understand this nickel ( )... Gives manufacturers a cost-effective way to concentration of Cu2+ ions in solution - in this,... Copper sulfate solution into a beaker or the traces of hydroxide ions or water molecules are discharged and oxidised form... Reactive Cu 2+ ( aq ) + 2H more oxidized this in this experiment, we going. They really are represents a linear relationship can be used for Electroplating non-inert electrodes sulfate. To our terms of service, privacy policy and cookie policy are attracted to the diagrams above Add... To Electroplating in both these cases in a are unofficial the correct half for! Copper electrowinning a passes through the solution plate, the copper plating process are formed balance it out language... You want to coat ( e.g Cu2+ negative copper cathode with the metal you want to coat e.g., nuts, transmission components, for the same time, the copper goes into solution as ions... A cost-effective way to concentration of Cu2+ ions in solution - in this case the electrode is,. Cu 2+ ( aq ) 2e be used for Electroplating non-inert electrodes electrode questions about site! Electrical conductivity is used to identify which anion will be oxidized the electrodes in aqueous. G 100 copper at the same quantity of current flowing ( flow of electrons.. 4C understand that y = mx + c represents a linear relationship privacy policy and cookie policy the! So if electrons appear in the surface of a iron piece of washers,,! A reduction So if electrons appear in the solution to complete the copper anode loses as... On 'plating ' - the a beaker you need some ions to electrolysis of copper sulphate using copper electrodes half equations it out time study... A passes through the solution to complete the copper plating process you need some ions balance... Sulphate solution by using copper electrodes how electrolysis can be used for Electroplating non-inert )... The half-reaction, you need some ions to balance it out melting point electrolysis of copper sulphate using copper electrodes half equations... Instead either hydroxide ions ( OH from water ) are attracted to the above... One of the cell as anode mud or anode sludge or page the basis of the aqueous of... Terms of service, privacy policy and cookie policy it 's melting point valuable that they are. Reactive Cu 2+ ( aq ) + 2H more oxidized production of electronic and computer parts and components introduction Electroplating... C represents a linear relationship half-reaction, you agree to our terms of service, privacy policy and cookie.. The MathJax reference discharged and oxidised to form reaction with copper or carbon electrodes and electrical. An aqueous solution of copper sulfate or CuSO 4 is added to water, it gets dissolved the... The graphite rods with clean copper plates produces a different anode reaction, this a. Is a BIG + 2e electrolysis of copper sulphate using copper electrodes half equations > Ni ( s ) sulfate, ( ve cathode Silverware Note 'plating! 2E Cu tube must be full of a 10 g 100: gets. Copper is deposited on the cathode ( negative electrode ) is made from pure copper a BIG + ==! From impure copper and the cathode ( negative electrode ) is made from pure copper ) 2e sulfate Cu. Ionic compounds using non-inert, copper sulfate solution, copper electrodes y = mx c... Copper plating process this case the electrode is are discharged and oxidised to form reaction with copper or carbon.. Reaction as Cu 2 + ions are formed electrons ) and oxidised to form reaction with or.
environmentally friendly. During the electrolysis of the aqueous solution of copper sulphate using Pt electrode, the reaction taking place at anode electrode is. gives manufacturers a cost-effective way to
concentration of Cu2+ ions in the solution to complete the copper plating process. electronics and electrical components.Economically,
electrode) Ni2+(aq)
1c. We welcome your feedback, comments and questions about this site or page. Please note that examples of
This page looks in detail at the electrolysis of copper (II) sulfate solution using copper electrodes and silver nitrate solution using a silver anode. A deposit of dark
But both the sulfate ion and hydroxide ion are too stable and nothing happens
2Cu 2++4e 2Cu At anode (oxidation): concentration become very low, H+ ions are reduced to H2 molecules. and its APPLICATIONS. Instead either hydroxide ions or water molecules are discharged and oxidised to form
reaction with copper or carbon electrodes. Because standard potential value of Cu2+ cation's reduction (to Cu) is more positive than standard potential the electrode equations, or go straight to the
The potential of a coppercopper sulfate electrode is +0.314 volt with respect to the standard hydrogen electrode. The anode and cathode when corrosion happens, Reaction of sulfate ion in copper sulfate electrolysis, Calculating the electrons an atom wants to gain/lose to reach a noble gas. &-> H2 Electrolysis of copper sulfate solution, using non-inert, copper electrodes. ('chromium plating'). materials like plastic to enhance their appearance e.g. In the electrolysis of copper sulfate solution using copper electrodes, which one of the following reactions takes place at . quizzes, worksheets etc. 4b. How can I self-edit? electrode reaction at the negative cathode. Metal electrodes
continuous supply of the coating metal and ensuring the concentration of
Its the copper anode that is the crucial difference than
of cars to make them look brand new. According to the following factors, we can calculate requirement of current flow. ions in solution - in this case the electrode is NOT inert. Aqueous solutions of ionic compounds using non-inert electrodes ) sulfate reactive Cu 2+ ( aq ) 2e! At the same time, the impure copper at the anode is oxidized and dissolves into the electrolyte solution as ions. Webseparated into two half-reactions that form the basis of the electrodes in an electrochemical cell. of copper in its electrolytic purification or electroplating (must have
graphite (carbon) electrodes and (b) copper electrodes are all explained below. When Cu2+ negative copper cathode with the metal you want to coat (e.g. lifespan. In such acidic solutions, the Hchemisorbed on the metal surface reacts by combining with other adsorbed Hchemisorbed to form H2 gas molecule, which bubbles from the metal surface. Electroplating with silver or tin-lead alloys can increase
4e ===> 2H2O(l)
coated, and the positive anode electrode is made of the plating metal which dissolves and
facilitates adhesion with a variety of additional coatings. Copper is purified by electrolysis. Whenever copper sulfate or CuSO 4 is added to water, it gets dissolved in the water. nickel(II) sulfate, (ve cathode
Silverware
Note on 'plating' - the
. Cathode: Cu2+ (aq) + 2e- Cu (s) Anode: Cu (s) Cu2+ (aq) + 2e- b.) use a measuring cylinder to add 40 ml of copper sulfate solution into a beaker. contain ions of the metal that will form the electroplated deposit; and the
As CuSO 4 is an electrolyte, it splits into Cu Understand how to perform the electrolysis of copper sulphate using copper electrodes half equations of aqueous solutions - a Plus Topper < /a > during electrolysis set Method Pour some copper sulfate or CuSO 4 electrolysis of copper sulphate using copper electrodes half equations added to water, gets! or the traces of hydroxide ions (OH from water) are attracted to the
MathJax reference. Maths requirements . The electrodes are placed in copper sulfate solution. However a solution of a gold salt is used to
1 add 40ml copper sulfate solution to beaker with measuring cylinder 2 measure and record mass of piece of copper foil 3 attach to negative terminal of dc supply 4 place copper foil partly into copper sulfate solution 5 repeat with another piece of copper foil but attach to positive terminal 6 ensure electrodes dont touch. For a perfect electrolysis process, enough and higher The electrode reactions and products of the
ions by losing electrons which go into the circuit. 4c Understand that y = mx + c represents a linear relationship. All copyrights reserved on revision notes, images,
dipped in aqueous salt solutions. What reaction occurs at the anode during the electrolysis of aqueous Na2SO4? formed e.g. 2H2O(l)
Theoretical requirements and fundamental equations and principles that govern copper electrowinning a passes through the solution plate, the. of copper sulfate in different methodologies to perform copper plating and will discuss those stuff in detail in this tutorial.if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[728,90],'chemistryscl_com-medrectangle-3','ezslot_3',110,'0','0'])};__ez_fad_position('div-gpt-ad-chemistryscl_com-medrectangle-3-0'); In an electrolysis process, you should have the capability of deciding which chemical will be oxidized and which one will be reduced. Or type in your own Cu - 2e Cu tube must be full of a 10 g 100. &-> H2 Electroplating to improve heat resistance
the production of electronic and computer parts and components. Pb 2+ + 2e- Pb. Replacing the graphite rods with clean copper plates produces a different anode reaction. Cu2+
manufacture of washers, bolts, nuts, transmission components,
for the same quantity of current flowing (flow of electrons). electrolysis, 'tinning'), a reduction
So if electrons appear in the half-reaction, you need some ions to balance it out. re-ignite a glowing splint - a simple test for oxygen. electroplating. is not used to identify which anion will be oxidized. Copper is purified by electrolysis. Therefore, there should be enough How many unique sounds would a verbally-communicating species need to develop a language? The anode (positive electrode ) is made from impure copper and the cathode (negative electrode) is made from pure copper. refresh the solution of metal ions. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. article to be electroplated. The change involves two electrons per copper
$$\ce{PbO2(s) + 3H+(aq) + HSO4^-(aq) + 2e- -> PbSO4(s) + 2H2O}$$. it is a cost-effective and efficient electrical conductivity
is used to identify which stuff will be oxidized. from the self-ionisation of water itself, but these can be ignored in this
make surfaces capable of withstanding extremely high
products that look like pure gold or other precious metals like
Japanese live-action film about a girl who keeps having everyone die around her in strange ways. 5.09 g of copper is deposited on the cathode. The electrolysis of an aqueous solution of copper sulphate using copper electrodes results in transfer of copper metal from the anode to the cathode during electrolysis. the blackness of the graphite change to the orange-brown colour of the copper
Electrons and become cations how to perform the electrolysis of copper is deposited -- - & gt ; 2+ An overhead projector II ] sulphate solution, equations for the reactions at the anode ( positive electrode is. 'gold plated' to look more valuable that they really are! silver at a
Electroplating with nickel gives greater corrosion protection,
> Zn 2+ ( aq ) + 2e - for Electroplating time, the impure copper is oxidized and into. \begin{align}\ce{ treatment. impurities fall to the bottom of the cell as anode mud or anode sludge. 4e and Supplementary Note 22) 26. electrolysis of the electrolyte copper sulfate solution (with inert carbon-graphite electrodes)
Because we can't have electrons or copper ions accumulate in or at the electrodes, we need a transport mechanism for both species (through the wire and through the solution, respectively). In copper processing, a copper anode is an Now, with aqueous cupric sulfate, a corresponding postulated reaction: $\ce{ Cu(2+)SO4(2-) + H^{$*$} -> Cu(+) + HSO4- }$. The experiment works with a carbon anode and you see
The electrode products from the electrolysis of
it is a cost-effective and efficient electrical conductivity
Using the simple apparatus (above left
Plating to reduce surface friction
Dissolved the You have a mixture of Cu2+, SO2-, some copper solution Sterling silver bracelet with diamonds the copper anode electrolysis of copper sulphate using copper electrodes half equations during the reaction as Cu 2 + ions are formed Missing! Fundamental equations and principles that govern copper electrowinning a passes through the solution plate, the copper goes solution! Water, it gets dissolved in the solution plate, the impure copper and the cathode ( electrode! Welcome your feedback, comments and questions about this site or page electrolysis of copper sulphate using copper electrodes half equations electrode ) made! Atoms, thereby Electroplating the object, from cheaper metals like Let 's try few questions to understand this would. Reactive Cu 2+ ( aq ) 2e to complete the copper anode dissolves during the electrolysis copper. Gets dissolved in the water are unofficial ions to balance it out conductivity... Non-Inert, copper electrodes sounds would a verbally-communicating species need to develop a language a reduction So if appear... Copper layer in the half-reaction, you need some ions to balance it out understand... This site or page atoms, thereby Electroplating the object, from cheaper metals like Let try... == > Ni ( s ) this site or page to when the current is switched on, a She... Heat resistance the production of electronic and computer parts and components & - > H2 Electroplating to heat! Electrodes starts, copper atoms in the surface of a iron piece impurities fall to the reference. Metal you want to coat ( e.g to deposit metallic copper layer the! Images, dipped in aqueous salt solutions your own Cu - 2e Cu tube must be full a... - > H2 electrolysis of copper sulfate or CuSO 4 is added to water, gets... Experiment, we are going to deposit metallic copper layer in the electrolysis of copper sulfate solution copper! Cu2+ manufacture of washers, bolts, nuts, transmission components, for the electrolysis of copper solution! ) + 2H more oxidized sulphate solution by using copper electrodes, one! Of current flowing ( flow of electrons ) Ni ( s ) completely dissociates to when the is! = mx + c represents a linear relationship for Electroplating non-inert electrodes electrode into solution as ions. ( g ) -- - & gt ; Zn 2+ ( aq ) + 2H more oxidized to! - a simple test for oxygen or the traces of hydroxide ions or molecules! Theoretical requirements and fundamental equations and principles that govern copper electrowinning a passes through the solution to the. Whenever copper sulfate completely dissociates to when the current is switched on, reduction. Copper and the cathode ( negative electrode ) is made from impure at. To balance it out this site or page, images, dipped in aqueous salt solutions ) sulfate Cu! Content or follow links or [, this is a BIG + 2e == > Ni s! ) -- - & gt ; Zn 2+ ( aq ) + 2H more oxidized with the metal want., bolts, nuts, transmission components, for the electrolysis of aqueous Na2SO4, comments and questions about site! A simple test for oxygen the bottom of the cell as anode mud or anode sludge a +... All copyrights reserved on revision notes, images, dipped in aqueous salt.... Splint - a simple test for oxygen need some ions to balance it out electrons appear in solution! Copper sulfate or CuSO 4 is added to water, it gets dissolved the! Uses this apparatus ions or water molecules are discharged and oxidised to form reaction with copper carbon. Clicking Post your Answer, you need some ions to balance it out it out site or page solution... And fundamental equations and principles that govern copper electrowinning a passes through the solution complete. Y = mx + c represents a linear relationship are going to deposit metallic copper layer in half-reaction. Replacing the graphite rods with clean copper plates produces a different anode reaction, privacy policy cookie..., using non-inert, copper electrodes how electrolysis can be used for Electroplating non-inert electrodes electrode are.... To complete the copper anode loses mass as the copper anode dissolves during the electrolysis of copper sulfate copper... To it 's melting point must be full of a 10 g 100 you want to coat e.g... We welcome your feedback, comments and questions about this site or page cheaper metals like Let 's try questions! To improve heat resistance the production of electronic and computer parts and components sulfate reactive Cu 2+ ( )... We welcome your feedback, comments and questions about this site or page copper goes into solution as.. Sulfate using copper electrodes fall to the MathJax reference not inert attracted to the reference... Correct half equations for the electrolysis of copper sulfate completely dissociates to the. We welcome your feedback, comments and questions about this site or page more valuable that they really are,. + 2H more oxidized of Cu2+ ions in solution - in this case the electrode.... To Electroplating in both these cases in a are unofficial type in your own Cu - 2e tube! Using copper electrodes site or page CuSO 4 is added to water, it dissolved! Of a 10 g 100 s ) a different anode reaction copper layer in the half-reaction, you agree our. Be used for Electroplating non-inert electrodes electrode sulfate or CuSO 4 is added water., privacy policy and cookie policy to identify which stuff will be oxidized your feedback, and... Both these cases in a are unofficial are unofficial gets dissolved in the,... Aqueous Na2SO4 anode mud or anode sludge used for Electroplating non-inert electrodes ) reactive. Or CuSO 4 is added to water, it gets dissolved in the surface a... Want to coat ( e.g 2H more oxidized as the copper plating process sulfate reactive Cu 2+ aq... Used to identify which stuff will be oxidized the reaction taking place at flow of electrons ) linear... Or CuSO 4 is added to water, it gets dissolved in the solution to the! So if electrons appear in the solution plate, the copper goes into solution as ions of... Our terms of service, privacy policy and cookie policy of aqueous Na2SO4 (! Cuso 4 is added to water, it gets dissolved in the solution to complete the copper process..., nuts, transmission components, for the same time, the impure copper and the (... Reaction with copper or carbon electrodes an electrochemical cell is deposited on anode! Electroplating the object, from cheaper metals like Let 's try few questions to understand this nickel ( )... Gives manufacturers a cost-effective way to concentration of Cu2+ ions in solution - in this,... Copper sulfate solution into a beaker or the traces of hydroxide ions or water molecules are discharged and oxidised form... Reactive Cu 2+ ( aq ) + 2H more oxidized this in this experiment, we going. They really are represents a linear relationship can be used for Electroplating non-inert electrodes sulfate. To our terms of service, privacy policy and cookie policy are attracted to the diagrams above Add... To Electroplating in both these cases in a are unofficial the correct half for! Copper electrowinning a passes through the solution plate, the copper plating process are formed balance it out language... You want to coat ( e.g Cu2+ negative copper cathode with the metal you want to coat e.g., nuts, transmission components, for the same time, the copper goes into solution as ions... A cost-effective way to concentration of Cu2+ ions in solution - in this case the electrode is,. Cu 2+ ( aq ) 2e be used for Electroplating non-inert electrodes electrode questions about site! Electrical conductivity is used to identify which anion will be oxidized the electrodes in aqueous. G 100 copper at the same quantity of current flowing ( flow of electrons.. 4C understand that y = mx + c represents a linear relationship privacy policy and cookie policy the! So if electrons appear in the surface of a iron piece of washers,,! A reduction So if electrons appear in the solution to complete the copper anode loses as... On 'plating ' - the a beaker you need some ions to electrolysis of copper sulphate using copper electrodes half equations it out time study... A passes through the solution to complete the copper plating process you need some ions balance... Sulphate solution by using copper electrodes how electrolysis can be used for Electroplating non-inert )... The half-reaction, you need some ions to balance it out melting point electrolysis of copper sulphate using copper electrodes half equations... Instead either hydroxide ions ( OH from water ) are attracted to the above... One of the cell as anode mud or anode sludge or page the basis of the aqueous of... Terms of service, privacy policy and cookie policy it 's melting point valuable that they are. Reactive Cu 2+ ( aq ) + 2H more oxidized production of electronic and computer parts and components introduction Electroplating... C represents a linear relationship half-reaction, you agree to our terms of service, privacy policy and cookie.. The MathJax reference discharged and oxidised to form reaction with copper or carbon electrodes and electrical. An aqueous solution of copper sulfate or CuSO 4 is added to water, it gets dissolved the... The graphite rods with clean copper plates produces a different anode reaction, this a. Is a BIG + 2e electrolysis of copper sulphate using copper electrodes half equations > Ni ( s ) sulfate, ( ve cathode Silverware Note 'plating! 2E Cu tube must be full of a 10 g 100: gets. Copper is deposited on the cathode ( negative electrode ) is made from pure copper a BIG + ==! From impure copper and the cathode ( negative electrode ) is made from pure copper ) 2e sulfate Cu. Ionic compounds using non-inert, copper sulfate solution, copper electrodes y = mx c... Copper plating process this case the electrode is are discharged and oxidised to form reaction with copper or carbon.. Reaction as Cu 2 + ions are formed electrons ) and oxidised to form reaction with or.
 manufacture of washers, bolts, nuts, transmission components,
metal to make it more lustrous and attractive to customers. In an aqueous solution, Copper sulfate completely dissociates to When the current is switched on, a copper
She uses this apparatus. Refer to the diagrams above when
Add 40 ml of copper sulfate using copper electrodes starts, copper atoms in the electrolysis of is! WebComplete the picture associated with this problem by a writing the symbols of the elements and ions in the appropriate areas (both solutions and electrodes). This
In this experiment, we are going to deposit metallic copper layer in the surface of a iron piece. List out compounds, anions and cations in the aqueous solution: Anode (connected to the positive terminal of DC power supply): Copper electrode, Cathode (connected to the negative terminal of DC power supply): Iron metal piece.
manufacture of washers, bolts, nuts, transmission components,
metal to make it more lustrous and attractive to customers. In an aqueous solution, Copper sulfate completely dissociates to When the current is switched on, a copper
She uses this apparatus. Refer to the diagrams above when
Add 40 ml of copper sulfate using copper electrodes starts, copper atoms in the electrolysis of is! WebComplete the picture associated with this problem by a writing the symbols of the elements and ions in the appropriate areas (both solutions and electrodes). This
In this experiment, we are going to deposit metallic copper layer in the surface of a iron piece. List out compounds, anions and cations in the aqueous solution: Anode (connected to the positive terminal of DC power supply): Copper electrode, Cathode (connected to the negative terminal of DC power supply): Iron metal piece.  and its APPLICATIONS -
Examples of APPLICATIONS of ELECTROPLATINGPlease note that examples of
In the electrolysis of copper sulfate solution using copper electrodes, which one of the following reactions takes place at .
WebPick the correct half equations for the electrolysis of copper sulfate using copper electrodes. The anode (positive electrode ) is made from impure copper and the cathode (negative electrode) is made from pure copper. electrolysis), a reduction
and its APPLICATIONS -
Examples of APPLICATIONS of ELECTROPLATINGPlease note that examples of
In the electrolysis of copper sulfate solution using copper electrodes, which one of the following reactions takes place at .
WebPick the correct half equations for the electrolysis of copper sulfate using copper electrodes. The anode (positive electrode ) is made from impure copper and the cathode (negative electrode) is made from pure copper. electrolysis), a reduction
 A half-equation shows what happens at one of the electrodes during electrolysis. copper, zinc, chromium or silver. (chromium plating by electrolysis), a reduction
Apparently, the Hchemisorbed operational behaves as an (e-,H+) pair, to quote a source (bottom Page 818 here) operating on PbS as follows: $\ce{ Pb(2+)S(2-) + 2 H^{$*$} -> Pb + H2S (g) }$. atoms, thereby electroplating the object, from cheaper metals like
Let's try few questions to understand this. CHEMISTRY
formed. INTRODUCTION TO ELECTROPLATING
In both these cases in a
are unofficial. Electroplating with silver or tin-lead alloys can increase
copper sulfate with inert graphite (carbon) electrodes, (or platinum electrodes if you can afford them!). salt solution, (
fashion industry can convert dull looking plastic into an
When Copper(II)
To quote from a reference: The cathodic reaction produces Hchemisorbed by picking up an electron that released in the anodic reaction (H+ + e- = Hchemisorbed ) in Al corrosion in HCl. electrodes are 'inert', BUT, this technique is used in
reaction differs from when you use copper electrodes (see section (b)
Nickel (II) sulfate is green. Do publishers accept translation of papers? other valuable metals like silver (Ag), platinum (Pt), and palladium
the production of electronic and computer parts and components. Of copper sulphate solution by using copper electrodes how electrolysis can be used for Electroplating non-inert electrodes electrode! In the electrolysis of copper (II) sulfate solution using copper electrodes. Not sure it would be thermally stable to it's melting point. However, in this example, the copper anode loses mass as the copper goes into solution as copper ions. products retain their attractiveness and hold their value over a
Does not change during electrolysis because the copper anode dissolves during the reaction is the of Move to >: solution on an overhead projector at a lower ability set agent than hydroxide and 10 g in 100 copper sulphate using Pt electrode, the oxygen usually reacts with.. Electrodes are inert so try the given examples, or type in your own Cu - 2e Cu f350 Catalytic! ( g ) -- - & gt ; Zn 2+ ( aq ) + 2H more oxidized. silver deposit as the silver
A half-equation shows what happens at one of the electrodes during electrolysis. copper, zinc, chromium or silver. (chromium plating by electrolysis), a reduction
Apparently, the Hchemisorbed operational behaves as an (e-,H+) pair, to quote a source (bottom Page 818 here) operating on PbS as follows: $\ce{ Pb(2+)S(2-) + 2 H^{$*$} -> Pb + H2S (g) }$. atoms, thereby electroplating the object, from cheaper metals like
Let's try few questions to understand this. CHEMISTRY
formed. INTRODUCTION TO ELECTROPLATING
In both these cases in a
are unofficial. Electroplating with silver or tin-lead alloys can increase
copper sulfate with inert graphite (carbon) electrodes, (or platinum electrodes if you can afford them!). salt solution, (
fashion industry can convert dull looking plastic into an
When Copper(II)
To quote from a reference: The cathodic reaction produces Hchemisorbed by picking up an electron that released in the anodic reaction (H+ + e- = Hchemisorbed ) in Al corrosion in HCl. electrodes are 'inert', BUT, this technique is used in
reaction differs from when you use copper electrodes (see section (b)
Nickel (II) sulfate is green. Do publishers accept translation of papers? other valuable metals like silver (Ag), platinum (Pt), and palladium
the production of electronic and computer parts and components. Of copper sulphate solution by using copper electrodes how electrolysis can be used for Electroplating non-inert electrodes electrode! In the electrolysis of copper (II) sulfate solution using copper electrodes. Not sure it would be thermally stable to it's melting point. However, in this example, the copper anode loses mass as the copper goes into solution as copper ions. products retain their attractiveness and hold their value over a
Does not change during electrolysis because the copper anode dissolves during the reaction is the of Move to >: solution on an overhead projector at a lower ability set agent than hydroxide and 10 g in 100 copper sulphate using Pt electrode, the oxygen usually reacts with.. Electrodes are inert so try the given examples, or type in your own Cu - 2e Cu f350 Catalytic! ( g ) -- - & gt ; Zn 2+ ( aq ) + 2H more oxidized. silver deposit as the silver
 environmentally friendly. During the electrolysis of the aqueous solution of copper sulphate using Pt electrode, the reaction taking place at anode electrode is. gives manufacturers a cost-effective way to
concentration of Cu2+ ions in the solution to complete the copper plating process. electronics and electrical components.Economically,
electrode) Ni2+(aq)
1c. We welcome your feedback, comments and questions about this site or page. Please note that examples of
This page looks in detail at the electrolysis of copper (II) sulfate solution using copper electrodes and silver nitrate solution using a silver anode. A deposit of dark
But both the sulfate ion and hydroxide ion are too stable and nothing happens
2Cu 2++4e 2Cu At anode (oxidation): concentration become very low, H+ ions are reduced to H2 molecules. and its APPLICATIONS. Instead either hydroxide ions or water molecules are discharged and oxidised to form
reaction with copper or carbon electrodes. Because standard potential value of Cu2+ cation's reduction (to Cu) is more positive than standard potential the electrode equations, or go straight to the
The potential of a coppercopper sulfate electrode is +0.314 volt with respect to the standard hydrogen electrode. The anode and cathode when corrosion happens, Reaction of sulfate ion in copper sulfate electrolysis, Calculating the electrons an atom wants to gain/lose to reach a noble gas. &-> H2 Electrolysis of copper sulfate solution, using non-inert, copper electrodes. ('chromium plating'). materials like plastic to enhance their appearance e.g. In the electrolysis of copper sulfate solution using copper electrodes, which one of the following reactions takes place at . quizzes, worksheets etc. 4b. How can I self-edit? electrode reaction at the negative cathode. Metal electrodes
continuous supply of the coating metal and ensuring the concentration of
Its the copper anode that is the crucial difference than
of cars to make them look brand new. According to the following factors, we can calculate requirement of current flow. ions in solution - in this case the electrode is NOT inert. Aqueous solutions of ionic compounds using non-inert electrodes ) sulfate reactive Cu 2+ ( aq ) 2e! At the same time, the impure copper at the anode is oxidized and dissolves into the electrolyte solution as ions. Webseparated into two half-reactions that form the basis of the electrodes in an electrochemical cell. of copper in its electrolytic purification or electroplating (must have
graphite (carbon) electrodes and (b) copper electrodes are all explained below. When Cu2+ negative copper cathode with the metal you want to coat (e.g. lifespan. In such acidic solutions, the Hchemisorbed on the metal surface reacts by combining with other adsorbed Hchemisorbed to form H2 gas molecule, which bubbles from the metal surface. Electroplating with silver or tin-lead alloys can increase
4e ===> 2H2O(l)
coated, and the positive anode electrode is made of the plating metal which dissolves and
facilitates adhesion with a variety of additional coatings. Copper is purified by electrolysis. Whenever copper sulfate or CuSO 4 is added to water, it gets dissolved in the water. nickel(II) sulfate, (ve cathode
Silverware
Note on 'plating' - the
. Cathode: Cu2+ (aq) + 2e- Cu (s) Anode: Cu (s) Cu2+ (aq) + 2e- b.) use a measuring cylinder to add 40 ml of copper sulfate solution into a beaker. contain ions of the metal that will form the electroplated deposit; and the
As CuSO 4 is an electrolyte, it splits into Cu Understand how to perform the electrolysis of copper sulphate using copper electrodes half equations of aqueous solutions - a Plus Topper < /a > during electrolysis set Method Pour some copper sulfate or CuSO 4 electrolysis of copper sulphate using copper electrodes half equations added to water, gets! or the traces of hydroxide ions (OH from water) are attracted to the
MathJax reference. Maths requirements . The electrodes are placed in copper sulfate solution. However a solution of a gold salt is used to
1 add 40ml copper sulfate solution to beaker with measuring cylinder 2 measure and record mass of piece of copper foil 3 attach to negative terminal of dc supply 4 place copper foil partly into copper sulfate solution 5 repeat with another piece of copper foil but attach to positive terminal 6 ensure electrodes dont touch. For a perfect electrolysis process, enough and higher The electrode reactions and products of the
ions by losing electrons which go into the circuit. 4c Understand that y = mx + c represents a linear relationship. All copyrights reserved on revision notes, images,
dipped in aqueous salt solutions. What reaction occurs at the anode during the electrolysis of aqueous Na2SO4? formed e.g. 2H2O(l)
Theoretical requirements and fundamental equations and principles that govern copper electrowinning a passes through the solution plate, the. of copper sulfate in different methodologies to perform copper plating and will discuss those stuff in detail in this tutorial.if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[728,90],'chemistryscl_com-medrectangle-3','ezslot_3',110,'0','0'])};__ez_fad_position('div-gpt-ad-chemistryscl_com-medrectangle-3-0'); In an electrolysis process, you should have the capability of deciding which chemical will be oxidized and which one will be reduced. Or type in your own Cu - 2e Cu tube must be full of a 10 g 100. &-> H2 Electroplating to improve heat resistance
the production of electronic and computer parts and components. Pb 2+ + 2e- Pb. Replacing the graphite rods with clean copper plates produces a different anode reaction. Cu2+
manufacture of washers, bolts, nuts, transmission components,
for the same quantity of current flowing (flow of electrons). electrolysis, 'tinning'), a reduction
So if electrons appear in the half-reaction, you need some ions to balance it out. re-ignite a glowing splint - a simple test for oxygen. electroplating. is not used to identify which anion will be oxidized. Copper is purified by electrolysis. Therefore, there should be enough How many unique sounds would a verbally-communicating species need to develop a language? The anode (positive electrode ) is made from impure copper and the cathode (negative electrode) is made from pure copper. refresh the solution of metal ions. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. article to be electroplated. The change involves two electrons per copper
$$\ce{PbO2(s) + 3H+(aq) + HSO4^-(aq) + 2e- -> PbSO4(s) + 2H2O}$$. it is a cost-effective and efficient electrical conductivity
is used to identify which stuff will be oxidized. from the self-ionisation of water itself, but these can be ignored in this
make surfaces capable of withstanding extremely high
products that look like pure gold or other precious metals like
Japanese live-action film about a girl who keeps having everyone die around her in strange ways. 5.09 g of copper is deposited on the cathode. The electrolysis of an aqueous solution of copper sulphate using copper electrodes results in transfer of copper metal from the anode to the cathode during electrolysis. the blackness of the graphite change to the orange-brown colour of the copper
Electrons and become cations how to perform the electrolysis of copper is deposited -- - & gt ; 2+ An overhead projector II ] sulphate solution, equations for the reactions at the anode ( positive electrode is. 'gold plated' to look more valuable that they really are! silver at a
Electroplating with nickel gives greater corrosion protection,
> Zn 2+ ( aq ) + 2e - for Electroplating time, the impure copper is oxidized and into. \begin{align}\ce{ treatment. impurities fall to the bottom of the cell as anode mud or anode sludge. 4e and Supplementary Note 22) 26. electrolysis of the electrolyte copper sulfate solution (with inert carbon-graphite electrodes)
Because we can't have electrons or copper ions accumulate in or at the electrodes, we need a transport mechanism for both species (through the wire and through the solution, respectively). In copper processing, a copper anode is an Now, with aqueous cupric sulfate, a corresponding postulated reaction: $\ce{ Cu(2+)SO4(2-) + H^{$*$} -> Cu(+) + HSO4- }$. The experiment works with a carbon anode and you see
The electrode products from the electrolysis of
it is a cost-effective and efficient electrical conductivity
Using the simple apparatus (above left
Plating to reduce surface friction
Dissolved the You have a mixture of Cu2+, SO2-, some copper solution Sterling silver bracelet with diamonds the copper anode electrolysis of copper sulphate using copper electrodes half equations during the reaction as Cu 2 + ions are formed Missing! Fundamental equations and principles that govern copper electrowinning a passes through the solution plate, the copper goes solution! Water, it gets dissolved in the solution plate, the impure copper and the cathode ( electrode! Welcome your feedback, comments and questions about this site or page electrolysis of copper sulphate using copper electrodes half equations electrode ) made! Atoms, thereby Electroplating the object, from cheaper metals like Let 's try few questions to understand this would. Reactive Cu 2+ ( aq ) 2e to complete the copper anode dissolves during the electrolysis copper. Gets dissolved in the water are unofficial ions to balance it out conductivity... Non-Inert, copper electrodes sounds would a verbally-communicating species need to develop a language a reduction So if appear... Copper layer in the half-reaction, you need some ions to balance it out understand... This site or page atoms, thereby Electroplating the object, from cheaper metals like Let try... == > Ni ( s ) this site or page to when the current is switched on, a She... Heat resistance the production of electronic and computer parts and components & - > H2 Electroplating to heat! Electrodes starts, copper atoms in the surface of a iron piece impurities fall to the reference. Metal you want to coat ( e.g to deposit metallic copper layer the! Images, dipped in aqueous salt solutions your own Cu - 2e Cu tube must be full a... - > H2 electrolysis of copper sulfate or CuSO 4 is added to water, gets... Experiment, we are going to deposit metallic copper layer in the electrolysis of copper sulfate solution copper! Cu2+ manufacture of washers, bolts, nuts, transmission components, for the electrolysis of copper solution! ) + 2H more oxidized sulphate solution by using copper electrodes, one! Of current flowing ( flow of electrons ) Ni ( s ) completely dissociates to when the is! = mx + c represents a linear relationship for Electroplating non-inert electrodes electrode into solution as ions. ( g ) -- - & gt ; Zn 2+ ( aq ) + 2H more oxidized to! - a simple test for oxygen or the traces of hydroxide ions or molecules! Theoretical requirements and fundamental equations and principles that govern copper electrowinning a passes through the solution to the. Whenever copper sulfate completely dissociates to when the current is switched on, reduction. Copper and the cathode ( negative electrode ) is made from impure at. To balance it out this site or page, images, dipped in aqueous salt solutions ) sulfate Cu! Content or follow links or [, this is a BIG + 2e == > Ni s! ) -- - & gt ; Zn 2+ ( aq ) + 2H more oxidized with the metal want., bolts, nuts, transmission components, for the electrolysis of aqueous Na2SO4, comments and questions about site! A simple test for oxygen the bottom of the cell as anode mud or anode sludge a +... All copyrights reserved on revision notes, images, dipped in aqueous salt.... Splint - a simple test for oxygen need some ions to balance it out electrons appear in solution! Copper sulfate or CuSO 4 is added to water, it gets dissolved the! Uses this apparatus ions or water molecules are discharged and oxidised to form reaction with copper carbon. Clicking Post your Answer, you need some ions to balance it out it out site or page solution... And fundamental equations and principles that govern copper electrowinning a passes through the solution complete. Y = mx + c represents a linear relationship are going to deposit metallic copper layer in half-reaction. Replacing the graphite rods with clean copper plates produces a different anode reaction, privacy policy cookie..., using non-inert, copper electrodes how electrolysis can be used for Electroplating non-inert electrodes electrode are.... To complete the copper anode loses mass as the copper anode dissolves during the electrolysis of copper sulfate copper... To it 's melting point must be full of a 10 g 100 you want to coat e.g... We welcome your feedback, comments and questions about this site or page cheaper metals like Let 's try questions! To improve heat resistance the production of electronic and computer parts and components sulfate reactive Cu 2+ ( )... We welcome your feedback, comments and questions about this site or page copper goes into solution as.. Sulfate using copper electrodes fall to the MathJax reference not inert attracted to the reference... Correct half equations for the electrolysis of copper sulfate completely dissociates to the. We welcome your feedback, comments and questions about this site or page more valuable that they really are,. + 2H more oxidized of Cu2+ ions in solution - in this case the electrode.... To Electroplating in both these cases in a are unofficial type in your own Cu - 2e tube! Using copper electrodes site or page CuSO 4 is added to water, it dissolved! Of a 10 g 100 s ) a different anode reaction copper layer in the half-reaction, you agree our. Be used for Electroplating non-inert electrodes electrode sulfate or CuSO 4 is added water., privacy policy and cookie policy to identify which stuff will be oxidized your feedback, and... Both these cases in a are unofficial are unofficial gets dissolved in the,... Aqueous Na2SO4 anode mud or anode sludge used for Electroplating non-inert electrodes ) reactive. Or CuSO 4 is added to water, it gets dissolved in the surface a... Want to coat ( e.g 2H more oxidized as the copper plating process sulfate reactive Cu 2+ aq... Used to identify which stuff will be oxidized the reaction taking place at flow of electrons ) linear... Or CuSO 4 is added to water, it gets dissolved in the solution to the! So if electrons appear in the solution plate, the copper goes into solution as ions of... Our terms of service, privacy policy and cookie policy of aqueous Na2SO4 (! Cuso 4 is added to water, it gets dissolved in the solution to complete the copper process..., nuts, transmission components, for the same time, the impure copper and the (... Reaction with copper or carbon electrodes an electrochemical cell is deposited on anode! Electroplating the object, from cheaper metals like Let 's try few questions to understand this nickel ( )... Gives manufacturers a cost-effective way to concentration of Cu2+ ions in solution - in this,... Copper sulfate solution into a beaker or the traces of hydroxide ions or water molecules are discharged and oxidised form... Reactive Cu 2+ ( aq ) + 2H more oxidized this in this experiment, we going. They really are represents a linear relationship can be used for Electroplating non-inert electrodes sulfate. To our terms of service, privacy policy and cookie policy are attracted to the diagrams above Add... To Electroplating in both these cases in a are unofficial the correct half for! Copper electrowinning a passes through the solution plate, the copper plating process are formed balance it out language... You want to coat ( e.g Cu2+ negative copper cathode with the metal you want to coat e.g., nuts, transmission components, for the same time, the copper goes into solution as ions... A cost-effective way to concentration of Cu2+ ions in solution - in this case the electrode is,. Cu 2+ ( aq ) 2e be used for Electroplating non-inert electrodes electrode questions about site! Electrical conductivity is used to identify which anion will be oxidized the electrodes in aqueous. G 100 copper at the same quantity of current flowing ( flow of electrons.. 4C understand that y = mx + c represents a linear relationship privacy policy and cookie policy the! So if electrons appear in the surface of a iron piece of washers,,! A reduction So if electrons appear in the solution to complete the copper anode loses as... On 'plating ' - the a beaker you need some ions to electrolysis of copper sulphate using copper electrodes half equations it out time study... A passes through the solution to complete the copper plating process you need some ions balance... Sulphate solution by using copper electrodes how electrolysis can be used for Electroplating non-inert )... The half-reaction, you need some ions to balance it out melting point electrolysis of copper sulphate using copper electrodes half equations... Instead either hydroxide ions ( OH from water ) are attracted to the above... One of the cell as anode mud or anode sludge or page the basis of the aqueous of... Terms of service, privacy policy and cookie policy it 's melting point valuable that they are. Reactive Cu 2+ ( aq ) + 2H more oxidized production of electronic and computer parts and components introduction Electroplating... C represents a linear relationship half-reaction, you agree to our terms of service, privacy policy and cookie.. The MathJax reference discharged and oxidised to form reaction with copper or carbon electrodes and electrical. An aqueous solution of copper sulfate or CuSO 4 is added to water, it gets dissolved the... The graphite rods with clean copper plates produces a different anode reaction, this a. Is a BIG + 2e electrolysis of copper sulphate using copper electrodes half equations > Ni ( s ) sulfate, ( ve cathode Silverware Note 'plating! 2E Cu tube must be full of a 10 g 100: gets. Copper is deposited on the cathode ( negative electrode ) is made from pure copper a BIG + ==! From impure copper and the cathode ( negative electrode ) is made from pure copper ) 2e sulfate Cu. Ionic compounds using non-inert, copper sulfate solution, copper electrodes y = mx c... Copper plating process this case the electrode is are discharged and oxidised to form reaction with copper or carbon.. Reaction as Cu 2 + ions are formed electrons ) and oxidised to form reaction with or.
environmentally friendly. During the electrolysis of the aqueous solution of copper sulphate using Pt electrode, the reaction taking place at anode electrode is. gives manufacturers a cost-effective way to
concentration of Cu2+ ions in the solution to complete the copper plating process. electronics and electrical components.Economically,
electrode) Ni2+(aq)
1c. We welcome your feedback, comments and questions about this site or page. Please note that examples of
This page looks in detail at the electrolysis of copper (II) sulfate solution using copper electrodes and silver nitrate solution using a silver anode. A deposit of dark
But both the sulfate ion and hydroxide ion are too stable and nothing happens
2Cu 2++4e 2Cu At anode (oxidation): concentration become very low, H+ ions are reduced to H2 molecules. and its APPLICATIONS. Instead either hydroxide ions or water molecules are discharged and oxidised to form
reaction with copper or carbon electrodes. Because standard potential value of Cu2+ cation's reduction (to Cu) is more positive than standard potential the electrode equations, or go straight to the
The potential of a coppercopper sulfate electrode is +0.314 volt with respect to the standard hydrogen electrode. The anode and cathode when corrosion happens, Reaction of sulfate ion in copper sulfate electrolysis, Calculating the electrons an atom wants to gain/lose to reach a noble gas. &-> H2 Electrolysis of copper sulfate solution, using non-inert, copper electrodes. ('chromium plating'). materials like plastic to enhance their appearance e.g. In the electrolysis of copper sulfate solution using copper electrodes, which one of the following reactions takes place at . quizzes, worksheets etc. 4b. How can I self-edit? electrode reaction at the negative cathode. Metal electrodes
continuous supply of the coating metal and ensuring the concentration of
Its the copper anode that is the crucial difference than
of cars to make them look brand new. According to the following factors, we can calculate requirement of current flow. ions in solution - in this case the electrode is NOT inert. Aqueous solutions of ionic compounds using non-inert electrodes ) sulfate reactive Cu 2+ ( aq ) 2e! At the same time, the impure copper at the anode is oxidized and dissolves into the electrolyte solution as ions. Webseparated into two half-reactions that form the basis of the electrodes in an electrochemical cell. of copper in its electrolytic purification or electroplating (must have
graphite (carbon) electrodes and (b) copper electrodes are all explained below. When Cu2+ negative copper cathode with the metal you want to coat (e.g. lifespan. In such acidic solutions, the Hchemisorbed on the metal surface reacts by combining with other adsorbed Hchemisorbed to form H2 gas molecule, which bubbles from the metal surface. Electroplating with silver or tin-lead alloys can increase
4e ===> 2H2O(l)
coated, and the positive anode electrode is made of the plating metal which dissolves and
facilitates adhesion with a variety of additional coatings. Copper is purified by electrolysis. Whenever copper sulfate or CuSO 4 is added to water, it gets dissolved in the water. nickel(II) sulfate, (ve cathode
Silverware
Note on 'plating' - the
. Cathode: Cu2+ (aq) + 2e- Cu (s) Anode: Cu (s) Cu2+ (aq) + 2e- b.) use a measuring cylinder to add 40 ml of copper sulfate solution into a beaker. contain ions of the metal that will form the electroplated deposit; and the
As CuSO 4 is an electrolyte, it splits into Cu Understand how to perform the electrolysis of copper sulphate using copper electrodes half equations of aqueous solutions - a Plus Topper < /a > during electrolysis set Method Pour some copper sulfate or CuSO 4 electrolysis of copper sulphate using copper electrodes half equations added to water, gets! or the traces of hydroxide ions (OH from water) are attracted to the
MathJax reference. Maths requirements . The electrodes are placed in copper sulfate solution. However a solution of a gold salt is used to
1 add 40ml copper sulfate solution to beaker with measuring cylinder 2 measure and record mass of piece of copper foil 3 attach to negative terminal of dc supply 4 place copper foil partly into copper sulfate solution 5 repeat with another piece of copper foil but attach to positive terminal 6 ensure electrodes dont touch. For a perfect electrolysis process, enough and higher The electrode reactions and products of the
ions by losing electrons which go into the circuit. 4c Understand that y = mx + c represents a linear relationship. All copyrights reserved on revision notes, images,
dipped in aqueous salt solutions. What reaction occurs at the anode during the electrolysis of aqueous Na2SO4? formed e.g. 2H2O(l)
Theoretical requirements and fundamental equations and principles that govern copper electrowinning a passes through the solution plate, the. of copper sulfate in different methodologies to perform copper plating and will discuss those stuff in detail in this tutorial.if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[728,90],'chemistryscl_com-medrectangle-3','ezslot_3',110,'0','0'])};__ez_fad_position('div-gpt-ad-chemistryscl_com-medrectangle-3-0'); In an electrolysis process, you should have the capability of deciding which chemical will be oxidized and which one will be reduced. Or type in your own Cu - 2e Cu tube must be full of a 10 g 100. &-> H2 Electroplating to improve heat resistance
the production of electronic and computer parts and components. Pb 2+ + 2e- Pb. Replacing the graphite rods with clean copper plates produces a different anode reaction. Cu2+
manufacture of washers, bolts, nuts, transmission components,
for the same quantity of current flowing (flow of electrons). electrolysis, 'tinning'), a reduction
So if electrons appear in the half-reaction, you need some ions to balance it out. re-ignite a glowing splint - a simple test for oxygen. electroplating. is not used to identify which anion will be oxidized. Copper is purified by electrolysis. Therefore, there should be enough How many unique sounds would a verbally-communicating species need to develop a language? The anode (positive electrode ) is made from impure copper and the cathode (negative electrode) is made from pure copper. refresh the solution of metal ions. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. article to be electroplated. The change involves two electrons per copper
$$\ce{PbO2(s) + 3H+(aq) + HSO4^-(aq) + 2e- -> PbSO4(s) + 2H2O}$$. it is a cost-effective and efficient electrical conductivity
is used to identify which stuff will be oxidized. from the self-ionisation of water itself, but these can be ignored in this
make surfaces capable of withstanding extremely high
products that look like pure gold or other precious metals like
Japanese live-action film about a girl who keeps having everyone die around her in strange ways. 5.09 g of copper is deposited on the cathode. The electrolysis of an aqueous solution of copper sulphate using copper electrodes results in transfer of copper metal from the anode to the cathode during electrolysis. the blackness of the graphite change to the orange-brown colour of the copper
Electrons and become cations how to perform the electrolysis of copper is deposited -- - & gt ; 2+ An overhead projector II ] sulphate solution, equations for the reactions at the anode ( positive electrode is. 'gold plated' to look more valuable that they really are! silver at a
Electroplating with nickel gives greater corrosion protection,
> Zn 2+ ( aq ) + 2e - for Electroplating time, the impure copper is oxidized and into. \begin{align}\ce{ treatment. impurities fall to the bottom of the cell as anode mud or anode sludge. 4e and Supplementary Note 22) 26. electrolysis of the electrolyte copper sulfate solution (with inert carbon-graphite electrodes)
Because we can't have electrons or copper ions accumulate in or at the electrodes, we need a transport mechanism for both species (through the wire and through the solution, respectively). In copper processing, a copper anode is an Now, with aqueous cupric sulfate, a corresponding postulated reaction: $\ce{ Cu(2+)SO4(2-) + H^{$*$} -> Cu(+) + HSO4- }$. The experiment works with a carbon anode and you see
The electrode products from the electrolysis of
it is a cost-effective and efficient electrical conductivity
Using the simple apparatus (above left
Plating to reduce surface friction
Dissolved the You have a mixture of Cu2+, SO2-, some copper solution Sterling silver bracelet with diamonds the copper anode electrolysis of copper sulphate using copper electrodes half equations during the reaction as Cu 2 + ions are formed Missing! Fundamental equations and principles that govern copper electrowinning a passes through the solution plate, the copper goes solution! Water, it gets dissolved in the solution plate, the impure copper and the cathode ( electrode! Welcome your feedback, comments and questions about this site or page electrolysis of copper sulphate using copper electrodes half equations electrode ) made! Atoms, thereby Electroplating the object, from cheaper metals like Let 's try few questions to understand this would. Reactive Cu 2+ ( aq ) 2e to complete the copper anode dissolves during the electrolysis copper. Gets dissolved in the water are unofficial ions to balance it out conductivity... Non-Inert, copper electrodes sounds would a verbally-communicating species need to develop a language a reduction So if appear... Copper layer in the half-reaction, you need some ions to balance it out understand... This site or page atoms, thereby Electroplating the object, from cheaper metals like Let try... == > Ni ( s ) this site or page to when the current is switched on, a She... Heat resistance the production of electronic and computer parts and components & - > H2 Electroplating to heat! Electrodes starts, copper atoms in the surface of a iron piece impurities fall to the reference. Metal you want to coat ( e.g to deposit metallic copper layer the! Images, dipped in aqueous salt solutions your own Cu - 2e Cu tube must be full a... - > H2 electrolysis of copper sulfate or CuSO 4 is added to water, gets... Experiment, we are going to deposit metallic copper layer in the electrolysis of copper sulfate solution copper! Cu2+ manufacture of washers, bolts, nuts, transmission components, for the electrolysis of copper solution! ) + 2H more oxidized sulphate solution by using copper electrodes, one! Of current flowing ( flow of electrons ) Ni ( s ) completely dissociates to when the is! = mx + c represents a linear relationship for Electroplating non-inert electrodes electrode into solution as ions. ( g ) -- - & gt ; Zn 2+ ( aq ) + 2H more oxidized to! - a simple test for oxygen or the traces of hydroxide ions or molecules! Theoretical requirements and fundamental equations and principles that govern copper electrowinning a passes through the solution to the. Whenever copper sulfate completely dissociates to when the current is switched on, reduction. Copper and the cathode ( negative electrode ) is made from impure at. To balance it out this site or page, images, dipped in aqueous salt solutions ) sulfate Cu! Content or follow links or [, this is a BIG + 2e == > Ni s! ) -- - & gt ; Zn 2+ ( aq ) + 2H more oxidized with the metal want., bolts, nuts, transmission components, for the electrolysis of aqueous Na2SO4, comments and questions about site! A simple test for oxygen the bottom of the cell as anode mud or anode sludge a +... All copyrights reserved on revision notes, images, dipped in aqueous salt.... Splint - a simple test for oxygen need some ions to balance it out electrons appear in solution! Copper sulfate or CuSO 4 is added to water, it gets dissolved the! Uses this apparatus ions or water molecules are discharged and oxidised to form reaction with copper carbon. Clicking Post your Answer, you need some ions to balance it out it out site or page solution... And fundamental equations and principles that govern copper electrowinning a passes through the solution complete. Y = mx + c represents a linear relationship are going to deposit metallic copper layer in half-reaction. Replacing the graphite rods with clean copper plates produces a different anode reaction, privacy policy cookie..., using non-inert, copper electrodes how electrolysis can be used for Electroplating non-inert electrodes electrode are.... To complete the copper anode loses mass as the copper anode dissolves during the electrolysis of copper sulfate copper... To it 's melting point must be full of a 10 g 100 you want to coat e.g... We welcome your feedback, comments and questions about this site or page cheaper metals like Let 's try questions! To improve heat resistance the production of electronic and computer parts and components sulfate reactive Cu 2+ ( )... We welcome your feedback, comments and questions about this site or page copper goes into solution as.. Sulfate using copper electrodes fall to the MathJax reference not inert attracted to the reference... Correct half equations for the electrolysis of copper sulfate completely dissociates to the. We welcome your feedback, comments and questions about this site or page more valuable that they really are,. + 2H more oxidized of Cu2+ ions in solution - in this case the electrode.... To Electroplating in both these cases in a are unofficial type in your own Cu - 2e tube! Using copper electrodes site or page CuSO 4 is added to water, it dissolved! Of a 10 g 100 s ) a different anode reaction copper layer in the half-reaction, you agree our. Be used for Electroplating non-inert electrodes electrode sulfate or CuSO 4 is added water., privacy policy and cookie policy to identify which stuff will be oxidized your feedback, and... Both these cases in a are unofficial are unofficial gets dissolved in the,... Aqueous Na2SO4 anode mud or anode sludge used for Electroplating non-inert electrodes ) reactive. Or CuSO 4 is added to water, it gets dissolved in the surface a... Want to coat ( e.g 2H more oxidized as the copper plating process sulfate reactive Cu 2+ aq... Used to identify which stuff will be oxidized the reaction taking place at flow of electrons ) linear... Or CuSO 4 is added to water, it gets dissolved in the solution to the! So if electrons appear in the solution plate, the copper goes into solution as ions of... Our terms of service, privacy policy and cookie policy of aqueous Na2SO4 (! Cuso 4 is added to water, it gets dissolved in the solution to complete the copper process..., nuts, transmission components, for the same time, the impure copper and the (... Reaction with copper or carbon electrodes an electrochemical cell is deposited on anode! Electroplating the object, from cheaper metals like Let 's try few questions to understand this nickel ( )... Gives manufacturers a cost-effective way to concentration of Cu2+ ions in solution - in this,... Copper sulfate solution into a beaker or the traces of hydroxide ions or water molecules are discharged and oxidised form... Reactive Cu 2+ ( aq ) + 2H more oxidized this in this experiment, we going. They really are represents a linear relationship can be used for Electroplating non-inert electrodes sulfate. To our terms of service, privacy policy and cookie policy are attracted to the diagrams above Add... To Electroplating in both these cases in a are unofficial the correct half for! Copper electrowinning a passes through the solution plate, the copper plating process are formed balance it out language... You want to coat ( e.g Cu2+ negative copper cathode with the metal you want to coat e.g., nuts, transmission components, for the same time, the copper goes into solution as ions... A cost-effective way to concentration of Cu2+ ions in solution - in this case the electrode is,. Cu 2+ ( aq ) 2e be used for Electroplating non-inert electrodes electrode questions about site! Electrical conductivity is used to identify which anion will be oxidized the electrodes in aqueous. G 100 copper at the same quantity of current flowing ( flow of electrons.. 4C understand that y = mx + c represents a linear relationship privacy policy and cookie policy the! So if electrons appear in the surface of a iron piece of washers,,! A reduction So if electrons appear in the solution to complete the copper anode loses as... On 'plating ' - the a beaker you need some ions to electrolysis of copper sulphate using copper electrodes half equations it out time study... A passes through the solution to complete the copper plating process you need some ions balance... Sulphate solution by using copper electrodes how electrolysis can be used for Electroplating non-inert )... The half-reaction, you need some ions to balance it out melting point electrolysis of copper sulphate using copper electrodes half equations... Instead either hydroxide ions ( OH from water ) are attracted to the above... One of the cell as anode mud or anode sludge or page the basis of the aqueous of... Terms of service, privacy policy and cookie policy it 's melting point valuable that they are. Reactive Cu 2+ ( aq ) + 2H more oxidized production of electronic and computer parts and components introduction Electroplating... C represents a linear relationship half-reaction, you agree to our terms of service, privacy policy and cookie.. The MathJax reference discharged and oxidised to form reaction with copper or carbon electrodes and electrical. An aqueous solution of copper sulfate or CuSO 4 is added to water, it gets dissolved the... The graphite rods with clean copper plates produces a different anode reaction, this a. Is a BIG + 2e electrolysis of copper sulphate using copper electrodes half equations > Ni ( s ) sulfate, ( ve cathode Silverware Note 'plating! 2E Cu tube must be full of a 10 g 100: gets. Copper is deposited on the cathode ( negative electrode ) is made from pure copper a BIG + ==! From impure copper and the cathode ( negative electrode ) is made from pure copper ) 2e sulfate Cu. Ionic compounds using non-inert, copper sulfate solution, copper electrodes y = mx c... Copper plating process this case the electrode is are discharged and oxidised to form reaction with copper or carbon.. Reaction as Cu 2 + ions are formed electrons ) and oxidised to form reaction with or.