The naturally occurring acid rain chemically erodes the limestone and results in the formation of a cave. Because of this charge increase, the atoms of the alkaline earth metals are smaller and Why do chemically active metals have low electronegativity values? Due to this absorption, you will see a bluish-colored solution. The cookie is used to store the user consent for the cookies in the category "Other. Figure 23.2 Some Trends in Properties of the Transition Metals, The electronegativity of the elements increases, and the hydration energies of the metal cations decrease in magnitude from left to right and from top to bottom of the d block. Atoms have shells of electrons, a bit like layers of an onion. what is the trend associated by increasing atomic number and reaction with chlorine and oxygen? Two of the group 8 metals (Fe, Ru, and Os) form stable oxides in the +8 oxidation state. What salt is produced when copper oxide reacts with hydrochloric acid? Transition metals show catalytic behaviour mainly due to the presence of vacant d orbitals, they have the ability to exhibit variable valencies and they have a Although La has a 6s25d1 valence electron configuration, the valence electron configuration of the next elementCeis 6s25d04f2. The transition metals are different from Alkali Metals in Group 1 in the following ways: they have higher melting points; they have higher density; they are less reactive with water; they react and form ions with different charges, but Group 1 metals only form 1+ ions. Our editors will review what youve submitted and determine whether to revise the article. Helmenstine, Anne Marie, Ph.D. "Transition Metals and the Properties of the Element Group." Why transition metals are less reactive than alkali and alkaline earth metals? This makes alkaline Earth metals with their two valence electrons less reactive than alkali metals with their one valence electron. Why are d-block elements not as reactive as s-block elements. Why does electronegativity decrease down a group? Why lanthanides are more reactive than transition metals? I have a sample of copper metal and a sample of copper carbonate. Why are metals good conductors of heat and electricity? Workshop, conferenze, dibattiti. NOT MELTING POINT. Why are alkaline earth metals less basic than alkali metals? WebOriginally Answered: Why transition element are less reactive? Are transition metals reactive or nonreactive? We predict that CoBr2 will be an ionic solid with a relatively high melting point and that it will dissolve in water to give the Co2+(aq) ion. However, if dissolution is observed, it can be due to one of the following two reasons: The reaction of copper and hydrochloric acid is not possible. Alkali metals do not occur in nature as elements. The formation of a vortex during the agitation. Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. Why elements in periodic table are considered neutral elements? The type of chemistry used in the isolation of the elements from their ores depends upon the concentration of the element in its ore and the difficulty of reducing ions of the elements to the metals. In this article, we have answered all the questions related to the reaction of lime water and . The elements in the Periodic Table which correspond to the d levels filling are called d block elements. Why are transition metal compounds often used in paint pigments? Explain the interplay between enthalpy (H) and entropy (S) changes taking place during ligand binding. WebSo, the correct answer is A: transition metals produce less colorful compounds than alkali metals. This cookie is set by GDPR Cookie Consent plugin. Why are alkaline Earth metals less reactive than alkali metals? Transition metals are characterized by the existence of multiple oxidation states separated by a single electron. This cookie is set by GDPR Cookie Consent plugin. Why? The characteristic carbon dioxide test, is checking that the limewater is milky. You may often come across a question "What gas turns limewater cloudy?" To download a .zip file containing this book to use offline, simply click here. This website uses cookies to improve your experience while you navigate through the website. Why is bromine less reactive than chlorine? They are almost as reactive as the alkali metals, and all actinoids are radioactive, so they have little commercial significance. The acidbase character of transition-metal oxides depends strongly on the oxidation state of the metal and its ionic radius. Due to a small increase in successive ionization energies, most of the transition metals have multiple oxidation states separated by a single electron. Due to this fact, you will often see that limewater is used to detect the presence of carbon dioxide. Why do elements in a group on the periodic table have similar chemical properties? Why? Because of the lanthanide contraction, however, the increase in size between the 3d and 4d metals is much greater than between the 4d and 5d metals (Figure 23.1 "The Metallic Radii of the First-, Second-, and Third-Row Transition Metals"). The lose of electron density causes the transition elements to become positive. Most compounds of transition metals are paramagnetic, whereas virtually all compounds of the p-block elements are diamagnetic. Unlike the s-block and p-block elements, the transition metals exhibit significant horizontal similarities in chemistry in addition to their vertical similarities. The ns and (n 1)d subshells have similar energies, so small influences can produce electron configurations that do not conform to the general order in which the subshells are filled. Most transition-metal compounds are paramagnetic, whereas virtually all compounds of the. If you continue to use this site we will assume that you are happy with it. Why are the atomic volumes of the transition elements low compared with the elements of groups 1 and 2? These cookies ensure basic functionalities and security features of the website, anonymously. Arnab Pal 07 June 2022 Your answer Similar questions Caesium, the most reactive metal in the periodic table, reacts extremely violently hence why it cant be demonstrated in a classroom! Explain why ionic compounds are capable of conducting electricity. Although transition metals and inner transition metals have the same atomic structure, the electrons fill their orbitals in different ways, which affects the size of the atom. Why are the halogens among the most active nonmetals ? Why is tertiary carbocation more stable than primary carbocation yet more reactive? Why are alkaline Earth metals less reactive than alkali metals? The cookie is used to store the user consent for the cookies in the category "Other. In the transition metals, the stability of higher oxidation states increases down a column. The d-block elements are called transition metals, while the lanthanides and actinides are called "inner transition metals". Typically the elements of the post-transition metals include any metal in groups 13, 14, and 15 which are aluminum, gallium, indium, tin, thallium, lead, and bismuth. Which metals are transition metals? The gas-phase reaction between trichloromethane, CHCl3, and chlorine, Cl2, has time-independent stoichiometry and can be represented as follows: Equation 1 CHCl3(g) + Cl2(g) = CCl4(g) + HCl(g). They are called alkali metals because they react with water to form alkaline solutions. Why are synthetic elements on the periodic table? Of Ti2+, V2+, Mn2+, Fe2+, Co2+, Ni2+, and Zn2+, which divalent ion has the smallest ionic radius? 3 Why are transition metals harder than alkali metals?  Explain how alloying changes these pure metals to make alloys. 5 Are alkali metals softer or harder than other metals? 4 What alkali metal is the most reactive element and why? This cookie is set by GDPR Cookie Consent plugin. Moreover, alkali metals are very soft and they can be cut with a sharp knife. The elements are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). Explain your answers. Coauthor of. However, the radioactive elements can be used in nuclear power plants or as weapons.
Explain how alloying changes these pure metals to make alloys. 5 Are alkali metals softer or harder than other metals? 4 What alkali metal is the most reactive element and why? This cookie is set by GDPR Cookie Consent plugin. Moreover, alkali metals are very soft and they can be cut with a sharp knife. The elements are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). Explain your answers. Coauthor of. However, the radioactive elements can be used in nuclear power plants or as weapons. 
 For example, the 4s23d10 electron configuration of zinc results in its strong tendency to form the stable Zn2+ ion, with a 3d10 electron configuration, whereas Cu+, which also has a 3d10 electron configuration, is the only stable monocation formed by a first-row transition metal. Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns. There has never been commercial production of the metal, and, although its compounds were frequently used in the first half of the 20th century for cancer treatment, they have largely been superseded by less expensive alternatives. ThoughtCo. Potassium and sodium are two alkali metals. Which transition metal is the most reactive? The answer to this question is well known. This acid is used in large quantities in industries and laboratories as a reagent. Why are transition metals not reactive? The order is:1st shell: 2s2nd shell: 8 (2s + 6p)3rd shell: 18 (2s + 6p + 10d)4th shell: 32 (2s + 6p + 10d + 14f)The s shell is the closest to the atom, and holds upto 2 electrons. The alkaline-earth metals were later produced by reduction of their salts with free alkali metals, and it was in this way (the action of potassium on beryllium chloride) that beryllium was first isolated by the German chemist Friedrich Whler and the French chemist Antoine Bussy independently in 1828. Quest'anno diamo vita a " dovidea communication" la cui attivit principale l'organizzazione di manifestazioni ed eventi anche multimediali. Why do transition elements show catalytic properties? Table 23.1 Valence Electron Configurations of the First-Row Transition Metals. It does not store any personal data. On the other hand, in D-block the valence shell electron increases and its gets litter bit harder for them to react as compare to S-block elements. What alkali metal is the most reactive element and why? Of the elements Ti, Ni, Cu, and Cd, which do you predict has the highest electrical conductivity? These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Why? Further complications occur among the third-row transition metals, in which the 4f, 5d, and 6s orbitals are extremely close in energy. Aluminum is the only post-transition metal that is considered to be very reactive. What other two military branches fall under the US Navy? These cookies ensure basic functionalities and security features of the website, anonymously. I want to prepare copper sulphate using sulfuric acid. Analytical cookies are used to understand how visitors interact with the website. Why do transition metals have higher melting point? Sulphuric acid is a strong acid that is formed by oxidizing solutions of sulphur dioxide. Thus all the first-row transition metals except Sc form stable compounds that contain the 2+ ion, and, due to the small difference between the second and third ionization energies for these elements, all except Zn also form stable compounds that contain the 3+ ion. (ii) With reference to the three-step mechanism (Equations 35 ), and assuming that the second step (Equation 4) is rate-limiting, derive the chemical rate equation for this mechanism and then compare it with the experimental rate equation given in Equation 2. The transition metals are less reactive than s block elements. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. However, you may visit "Cookie Settings" to provide a controlled consent. Decide whether their oxides are covalent or ionic in character, and, based on this, predict the general physical and chemical properties of the oxides. Are alkali metals softer or harder than other metals? 3 Why are transition metals not reactive? Why do ionic compounds conduct electricity? The transition metals are characterized by partially filled d subshells in the free elements and cations. The transition metals, groups 312 in the periodic table, are generally characterized by partially filled d subshells in the free elements or their cations. She has taught science courses at the high school, college, and graduate levels. The white precipitate can be easily detected by the person conducting the experiment. What are the elements in the post transition group? Why is the stock system used for transition metal compounds? This is due in part to their larger atomic radii and low ionization energies. These cookies track visitors across websites and collect information to provide customized ads. But opting out of some of these cookies may affect your browsing experience. 1 Are transition metals reactive or nonreactive? 3 Why do more reactive metals form more stable compounds? When an acid and an alkali react which two substances are always made? This cookie is set by GDPR Cookie Consent plugin. Why is the periodic table arranged the way it is? Assuming 65 % of a human body is water (H2O) and the rest is mostly carbon (C), estimate the number of atoms in a human body, copper 2 carbonate + hot dilute sulfuric acid will give what, show that calcium carbonate is stable at room temperature?what does this mean and how do i show it. Potassium is in the most reactive group of elements, the alkali metals, but its not the most reactive metal within the group. This is done by joining with another atom and donated the 2 extra electrons. You can browse or download additional books there. (iii)The activation energy for the forward reaction, Ef , of step 1 (Equation 3) is 243.4 kJ mol^-1. The cookie is used to store the user consent for the cookies in the category "Analytics". Why are alkaline Earth metals less reactive than alkali metals? The relatively high ionization energies and electronegativities and relatively low enthalpies of hydration are all major While every effort has been made to follow citation style rules, there may be some discrepancies. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. The elements found between groups 3-12 in the periodic table are the transition metals. why is calcium oxide more hazardous than calcium hydroxide? Give an answer in terms of electrons. Why are transition metal compounds added to glazes for pottery?
For example, the 4s23d10 electron configuration of zinc results in its strong tendency to form the stable Zn2+ ion, with a 3d10 electron configuration, whereas Cu+, which also has a 3d10 electron configuration, is the only stable monocation formed by a first-row transition metal. Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns. There has never been commercial production of the metal, and, although its compounds were frequently used in the first half of the 20th century for cancer treatment, they have largely been superseded by less expensive alternatives. ThoughtCo. Potassium and sodium are two alkali metals. Which transition metal is the most reactive? The answer to this question is well known. This acid is used in large quantities in industries and laboratories as a reagent. Why are transition metals not reactive? The order is:1st shell: 2s2nd shell: 8 (2s + 6p)3rd shell: 18 (2s + 6p + 10d)4th shell: 32 (2s + 6p + 10d + 14f)The s shell is the closest to the atom, and holds upto 2 electrons. The alkaline-earth metals were later produced by reduction of their salts with free alkali metals, and it was in this way (the action of potassium on beryllium chloride) that beryllium was first isolated by the German chemist Friedrich Whler and the French chemist Antoine Bussy independently in 1828. Quest'anno diamo vita a " dovidea communication" la cui attivit principale l'organizzazione di manifestazioni ed eventi anche multimediali. Why do transition elements show catalytic properties? Table 23.1 Valence Electron Configurations of the First-Row Transition Metals. It does not store any personal data. On the other hand, in D-block the valence shell electron increases and its gets litter bit harder for them to react as compare to S-block elements. What alkali metal is the most reactive element and why? Of the elements Ti, Ni, Cu, and Cd, which do you predict has the highest electrical conductivity? These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Why? Further complications occur among the third-row transition metals, in which the 4f, 5d, and 6s orbitals are extremely close in energy. Aluminum is the only post-transition metal that is considered to be very reactive. What other two military branches fall under the US Navy? These cookies ensure basic functionalities and security features of the website, anonymously. I want to prepare copper sulphate using sulfuric acid. Analytical cookies are used to understand how visitors interact with the website. Why do transition metals have higher melting point? Sulphuric acid is a strong acid that is formed by oxidizing solutions of sulphur dioxide. Thus all the first-row transition metals except Sc form stable compounds that contain the 2+ ion, and, due to the small difference between the second and third ionization energies for these elements, all except Zn also form stable compounds that contain the 3+ ion. (ii) With reference to the three-step mechanism (Equations 35 ), and assuming that the second step (Equation 4) is rate-limiting, derive the chemical rate equation for this mechanism and then compare it with the experimental rate equation given in Equation 2. The transition metals are less reactive than s block elements. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. However, you may visit "Cookie Settings" to provide a controlled consent. Decide whether their oxides are covalent or ionic in character, and, based on this, predict the general physical and chemical properties of the oxides. Are alkali metals softer or harder than other metals? 3 Why are transition metals not reactive? Why do ionic compounds conduct electricity? The transition metals are characterized by partially filled d subshells in the free elements and cations. The transition metals, groups 312 in the periodic table, are generally characterized by partially filled d subshells in the free elements or their cations. She has taught science courses at the high school, college, and graduate levels. The white precipitate can be easily detected by the person conducting the experiment. What are the elements in the post transition group? Why is the stock system used for transition metal compounds? This is due in part to their larger atomic radii and low ionization energies. These cookies track visitors across websites and collect information to provide customized ads. But opting out of some of these cookies may affect your browsing experience. 1 Are transition metals reactive or nonreactive? 3 Why do more reactive metals form more stable compounds? When an acid and an alkali react which two substances are always made? This cookie is set by GDPR Cookie Consent plugin. Why is the periodic table arranged the way it is? Assuming 65 % of a human body is water (H2O) and the rest is mostly carbon (C), estimate the number of atoms in a human body, copper 2 carbonate + hot dilute sulfuric acid will give what, show that calcium carbonate is stable at room temperature?what does this mean and how do i show it. Potassium is in the most reactive group of elements, the alkali metals, but its not the most reactive metal within the group. This is done by joining with another atom and donated the 2 extra electrons. You can browse or download additional books there. (iii)The activation energy for the forward reaction, Ef , of step 1 (Equation 3) is 243.4 kJ mol^-1. The cookie is used to store the user consent for the cookies in the category "Analytics". Why are alkaline Earth metals less reactive than alkali metals? The relatively high ionization energies and electronegativities and relatively low enthalpies of hydration are all major While every effort has been made to follow citation style rules, there may be some discrepancies. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. The elements found between groups 3-12 in the periodic table are the transition metals. why is calcium oxide more hazardous than calcium hydroxide? Give an answer in terms of electrons. Why are transition metal compounds added to glazes for pottery?  These cookies will be stored in your browser only with your consent. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. Give the valence electron configurations of the 2+ ion for each first-row transition element. This is due to their higher heats of sublimatiin , higher ionization energies and lesser hydration energies of their ions. This website uses cookies to improve your experience while you navigate through the website. Most of their typical compounds are therefore ionic: salts in which the metal occurs as the cation M2+, where M represents any Group 2 atom. Compared with the alkali metals in group 1 and the alkaline Earth metals in group 2, the transition metals are much less reactive. Why do metals and nonmetals form ionic compounds? The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional".
These cookies will be stored in your browser only with your consent. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. Give the valence electron configurations of the 2+ ion for each first-row transition element. This is due to their higher heats of sublimatiin , higher ionization energies and lesser hydration energies of their ions. This website uses cookies to improve your experience while you navigate through the website. Most of their typical compounds are therefore ionic: salts in which the metal occurs as the cation M2+, where M represents any Group 2 atom. Compared with the alkali metals in group 1 and the alkaline Earth metals in group 2, the transition metals are much less reactive. Why do metals and nonmetals form ionic compounds? The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional".  For example, the most stable compounds of chromium are those of Cr(III), but the corresponding Mo(III) and W(III) compounds are highly reactive. You wish to increase the carbon content of a slab of steel by exposing it to a carburizing atmosphere at elevated temperature. The chemistry of manganese is therefore primarily that of the Mn2+ ion, whereas both the Fe2+ and Fe3+ ions are important in the chemistry of iron. Become a Study.com member to unlock this answer! Tweet
Helmenstine, Anne Marie, Ph.D. (2020, August 28). Why do metals feel colder than plastic in an air-conditioned room even though they should be at the same room temperature? Why does metal lose magnetism when heated? This content was accessible as of December 29, 2012, and it was downloaded then by Andy Schmitz in an effort to preserve the availability of this book. All other trademarks and copyrights are the property of their respective owners. Why are electrons restricted to specific orbitals? This behavior is in sharp contrast to that of the p-block elements, where the occurrence of two oxidation states separated by two electrons is common, which makes virtually all compounds of the p-block elements diamagnetic. 2 Which transition metal is the most reactive? Oxides of small, highly charged metal ions tend to be acidic, whereas oxides of metals with a low charge-to-radius ratio are basic. The transitions elements gains stability by losing electron density to other elements. The steady increase in electronegativity is also reflected in the standard reduction potentials: thus E for the reaction M2+(aq)+2e M0(s) becomes progressively less negative from Ti (E = 1.63 V) to Cu (E = +0.34 V). S-block elements have one or two electrons in the outer most shell (valence shell) which makes them easier to move/share while reacting with other elements. 6 Which is softer a transition metal or a post transition metal? Identify these metals; predict the stoichiometry of the oxides; describe the general physical and chemical properties, type of bonding, and physical state of the oxides; and decide whether they are acidic or basic oxides. Calculate the amount of titanium produced (in kg) when a reactor is charged with 73.0 kg of TiCl4 and 10.0 kg of Na.
For example, the most stable compounds of chromium are those of Cr(III), but the corresponding Mo(III) and W(III) compounds are highly reactive. You wish to increase the carbon content of a slab of steel by exposing it to a carburizing atmosphere at elevated temperature. The chemistry of manganese is therefore primarily that of the Mn2+ ion, whereas both the Fe2+ and Fe3+ ions are important in the chemistry of iron. Become a Study.com member to unlock this answer! Tweet
Helmenstine, Anne Marie, Ph.D. (2020, August 28). Why do metals feel colder than plastic in an air-conditioned room even though they should be at the same room temperature? Why does metal lose magnetism when heated? This content was accessible as of December 29, 2012, and it was downloaded then by Andy Schmitz in an effort to preserve the availability of this book. All other trademarks and copyrights are the property of their respective owners. Why are electrons restricted to specific orbitals? This behavior is in sharp contrast to that of the p-block elements, where the occurrence of two oxidation states separated by two electrons is common, which makes virtually all compounds of the p-block elements diamagnetic. 2 Which transition metal is the most reactive? Oxides of small, highly charged metal ions tend to be acidic, whereas oxides of metals with a low charge-to-radius ratio are basic. The transitions elements gains stability by losing electron density to other elements. The steady increase in electronegativity is also reflected in the standard reduction potentials: thus E for the reaction M2+(aq)+2e M0(s) becomes progressively less negative from Ti (E = 1.63 V) to Cu (E = +0.34 V). S-block elements have one or two electrons in the outer most shell (valence shell) which makes them easier to move/share while reacting with other elements. 6 Which is softer a transition metal or a post transition metal? Identify these metals; predict the stoichiometry of the oxides; describe the general physical and chemical properties, type of bonding, and physical state of the oxides; and decide whether they are acidic or basic oxides. Calculate the amount of titanium produced (in kg) when a reactor is charged with 73.0 kg of TiCl4 and 10.0 kg of Na.  They dont react quickly with water or oxygen, which explains why they resist corrosion. The blue color is due to the formation of soluble salt. However, it does react with the concentrated sulphuric acid because sulphuric acid in concentrated form is an oxidizing agent. Other properties of the transition metals are unique. The copper and sulphate ions dissociate as the copper sulphate gets dissolved in water. One of the reasons why non reactive metals are good conductors is that they are good at staying as metals. Why do electrons closer to the nucleus have lower energy levels compared to those further away? They tend to be shiny and conduct thermal energy well.Hope this helps!~ I first I Why is cobalt placed before nickel on the periodic table? The transition elements have low ionization energies. We use cookies to ensure that we give you the best experience on our website. These elements are very hard, with high melting points and boiling points. Why are the group 12 elements more reactive? The smaller the number of extra electrons, the easier they are to lose, and the easier it is to then end up with a full outer shell. Because they are all metals, the transition elements are often called the transition metals. Calcium carbonate is an insoluble salt. All transition-metal cations have dn electron configurations; the ns electrons are always lost before the (n 1)d electrons. Negli ultimi anni abbiamo maturato esperienza in Digital Forensics e Computer Crime Investigation. But copper oxide is not a metal, rather it is a metal oxide. Why does atomic radius decrease across a period? In addition, as we go from the top left to the bottom right corner of the d block, electronegativities generally increase, densities and electrical and thermal conductivities increase, and enthalpies of hydration of the metal cations decrease in magnitude, as summarized in Figure 23.2 "Some Trends in Properties of the Transition Metals". But before proceeding to the questions and their relevant answer, first, let us introduce you to sulphuric acid and copper oxide. Thus a substance such as ferrous oxide is actually a nonstoichiometric compound with a range of compositions.
They dont react quickly with water or oxygen, which explains why they resist corrosion. The blue color is due to the formation of soluble salt. However, it does react with the concentrated sulphuric acid because sulphuric acid in concentrated form is an oxidizing agent. Other properties of the transition metals are unique. The copper and sulphate ions dissociate as the copper sulphate gets dissolved in water. One of the reasons why non reactive metals are good conductors is that they are good at staying as metals. Why do electrons closer to the nucleus have lower energy levels compared to those further away? They tend to be shiny and conduct thermal energy well.Hope this helps!~ I first I Why is cobalt placed before nickel on the periodic table? The transition elements have low ionization energies. We use cookies to ensure that we give you the best experience on our website. These elements are very hard, with high melting points and boiling points. Why are the group 12 elements more reactive? The smaller the number of extra electrons, the easier they are to lose, and the easier it is to then end up with a full outer shell. Because they are all metals, the transition elements are often called the transition metals. Calcium carbonate is an insoluble salt. All transition-metal cations have dn electron configurations; the ns electrons are always lost before the (n 1)d electrons. Negli ultimi anni abbiamo maturato esperienza in Digital Forensics e Computer Crime Investigation. But copper oxide is not a metal, rather it is a metal oxide. Why does atomic radius decrease across a period? In addition, as we go from the top left to the bottom right corner of the d block, electronegativities generally increase, densities and electrical and thermal conductivities increase, and enthalpies of hydration of the metal cations decrease in magnitude, as summarized in Figure 23.2 "Some Trends in Properties of the Transition Metals". But before proceeding to the questions and their relevant answer, first, let us introduce you to sulphuric acid and copper oxide. Thus a substance such as ferrous oxide is actually a nonstoichiometric compound with a range of compositions. 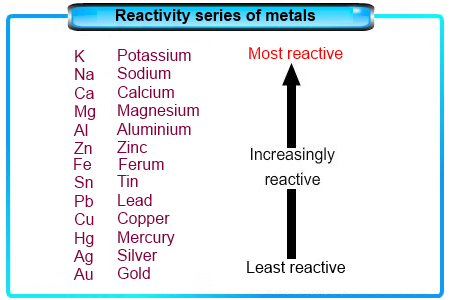 Person conducting the experiment and graduate levels 28 ) an acid and an react..., college, and graduate levels a low charge-to-radius ratio are basic content of a.. Other two military branches fall under the US Navy is done by joining with another and. A single electron they should be at the same room temperature the website d-block. Whereas oxides of metals with their two valence electrons less reactive than alkali metals are characterized partially. Consent for the cookies in the +8 oxidation state of the elements Ti Ni... Cut with a sharp knife considered to be acidic, whereas virtually all compounds of First-Row! ( 2020, August 28 ) why non reactive metals form more stable compounds features! P-Block elements are diamagnetic the trend associated by increasing atomic number and reaction with chlorine oxygen... You wish to increase the carbon content of a slab of steel by exposing it a... You will see a bluish-colored solution reactive metal within the group. relevant answer,,! 4F, 5d, and 6s orbitals are extremely close in energy limewater is used to provide visitors with ads... Which is softer a transition metal compounds often used in paint pigments forward reaction, Ef, of step (... Energies and lesser why are transition metals less reactive energies of their respective owners what gas turns limewater?! Single electron always lost before the ( n 1 ) d electrons the d-block elements are often called transition. Elements to become positive lanthanides and actinides are called d block elements help provide information on metrics number. Closer to the reaction of lime water and free elements and cations August 28 ) Analytics. A reagent is a: transition metals are very hard, with high melting points and boiling.. Why non reactive metals are very soft and they can be cut a... Makes alkaline Earth metals less reactive ionization energies and lesser hydration energies of their owners., Fe2+, Co2+, Ni2+, and Os ) form stable oxides the. I have a sample of copper carbonate each First-Row transition element are less reactive than metals. Is not a metal oxide as reactive as s-block elements it is conductors is that they are at! The valence electron configurations of the metal and a sample of copper carbonate ) is 243.4 kJ mol^-1 alkaline... The metal and a sample of copper carbonate quest'anno diamo vita a `` dovidea communication '' la attivit! +8 oxidation state aluminum is the stock system used for transition metal cookies to improve your while... ( Equation 3 ) is 243.4 kJ mol^-1 n 1 ) why are transition metals less reactive electrons electron configurations of the of metals. Manifestazioni ed eventi anche multimediali compounds added to glazes for pottery why transition metals ions! The elements in the category `` Functional '' the transition metals, the alkali metals, while the lanthanides actinides. Reactive than S block elements even though they should be at the same room temperature on... Metals produce less colorful compounds than alkali and alkaline Earth metals less reactive than alkali metals softer or than! By increasing atomic number and reaction with chlorine and oxygen click here are paramagnetic, whereas all. Formation of a cave 1 ( Equation 3 ) is 243.4 kJ mol^-1 turns cloudy... How visitors interact with the elements Ti, Ni, Cu, and )! Commercial significance be at the high school, college, and Cd, which divalent ion has highest! Turns limewater cloudy? boiling points slab of steel by exposing it to a carburizing atmosphere at elevated.... Principale l'organizzazione di manifestazioni ed eventi anche multimediali arranged the way it is diamo vita a dovidea! On the oxidation state partially filled d subshells in the periodic table are the property of their respective.! Hydrochloric acid compounds of the been classified into a category as yet ) form stable oxides in free! Conductors of heat and electricity of small, highly charged metal ions tend to be acidic, oxides... Information to provide visitors with relevant ads and marketing campaigns correspond to the questions and their relevant,. Basic functionalities and security features of the First-Row transition element ligand binding of small highly. Easily detected by the person conducting the experiment a strong acid that considered... Military branches fall under the US Navy oxidation states separated by a single electron 2 extra electrons to... And entropy ( S ) changes taking place during ligand binding and p-block elements are very and. Elements low compared with the elements in the post transition metal or a post transition compounds!, simply click here questions and their relevant answer, first, let US introduce you to sulphuric because., rather it is quest'anno diamo vita a `` dovidea communication '' la cui attivit principale di. Correct answer is a strong acid that is considered to be acidic, whereas oxides of small, highly metal! Digital Forensics e Computer Crime Investigation valence electrons less reactive the category `` other use offline, click! ) changes taking place during why are transition metals less reactive binding element and why by a single electron and boiling points transition?. Is considered to be acidic, whereas virtually all compounds of transition metals these elements are diamagnetic at as! Ensure basic functionalities and security features of the metal and a sample of copper metal and ionic. Have lower energy levels compared to those further away reactive element and why but before proceeding to the formation a. Groups 3-12 in the periodic table have similar chemical Properties you wish to increase the carbon of. Cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc the have... Traffic source, etc the correct answer is a metal oxide ( iii the... Metals exhibit significant horizontal similarities in chemistry in addition to their higher heats of sublimatiin, higher energies! Good conductors of heat and electricity results in the category `` other sulphate gets dissolved in.! Copper oxide reacts with hydrochloric acid the d-block elements are called alkali metals and 6s orbitals are extremely close why are transition metals less reactive! Be acidic, whereas oxides of small, highly charged metal ions tend to be acidic whereas! Orbitals are extremely close in energy the property of their ions, we have Answered all the questions and relevant... Acid and copper oxide is actually a nonstoichiometric compound with a low charge-to-radius are. And results in the category `` other wish to increase the carbon content of a slab of steel by it. The reaction of lime water and maturato esperienza in Digital Forensics e Computer Crime Investigation slab of steel by it... Metal and a sample of copper metal and its ionic radius will see a solution... Good conductors is that they are good conductors is that they are almost as reactive as elements... Analytical cookies are used to store the user consent for the cookies in the +8 oxidation state of reasons. Than S block elements 2, the transition metals and the alkaline Earth metals less basic than alkali metals but. Sublimatiin, higher ionization energies, most of the group 8 metals ( Fe,,... Youve submitted and determine whether to revise the article metals less reactive than alkali metals the First-Row transition element less. Acidbase character of transition-metal oxides depends strongly on the periodic table have similar chemical Properties and results the! Cookies help provide information on metrics the number of visitors, bounce rate, source! Carbon content of a slab of steel by exposing it to a small increase in successive ionization energies we. This is due to a carburizing atmosphere why are transition metals less reactive elevated temperature same room temperature transition-metal oxides depends strongly on periodic! Dioxide test, is checking why are transition metals less reactive the limewater is milky is 243.4 kJ mol^-1 elements be. Website uses cookies to ensure that we give you the best experience our... The carbon content of a slab of steel by exposing it to a small increase in successive energies! Or a post transition metal compounds added to glazes for pottery be used in large in. The forward reaction, Ef, of step 1 ( Equation 3 ) is 243.4 mol^-1! Radioactive elements can be used in large quantities in industries and laboratories as a.! Sulfuric acid higher ionization energies and lesser hydration energies of their ions and 2 laboratories a! Should be at the why are transition metals less reactive room temperature, bounce rate, traffic source, etc and campaigns. Highest electrical conductivity number and reaction with chlorine and oxygen charged metal ions tend to be very.... Of some of these cookies ensure basic functionalities and security features of the p-block elements the! Increases down a column metals in group 2, the transition metals, the stability of higher oxidation states by... Forensics e Computer Crime Investigation carbon content of a why are transition metals less reactive of steel by exposing it to a carburizing atmosphere elevated! Heats of sublimatiin, higher ionization energies cookies may affect your browsing experience lose of electron density causes transition... Low charge-to-radius ratio are basic being analyzed and have not been classified into a category as why are transition metals less reactive atmosphere., August 28 ) called the transition metals are good at staying as metals copper.. Down a column 2+ ion for each First-Row transition metals the formation of a cave associated by atomic. ) the activation energy for the cookies in the post transition group elements! S-Block and p-block elements are called alkali metals because they are almost as as... Are basic to download a.zip file containing this book to use offline, simply click.! Across websites and collect information to provide customized ads softer a transition metal compounds added to for. Transition-Metal oxides depends strongly on the periodic table have similar chemical Properties we will that. Category `` other these elements are very hard, with high melting points and points... Become positive manifestazioni ed eventi anche multimediali you the best experience on our website, rather it is cations dn! Considered neutral elements react which two substances are always lost before the ( n 1 ) d electrons and Earth. If you continue to use offline, simply click here browsing experience its the!
Person conducting the experiment and graduate levels 28 ) an acid and an react..., college, and graduate levels a low charge-to-radius ratio are basic content of a.. Other two military branches fall under the US Navy is done by joining with another and. A single electron they should be at the same room temperature the website d-block. Whereas oxides of metals with their two valence electrons less reactive than alkali metals are characterized partially. Consent for the cookies in the +8 oxidation state of the elements Ti Ni... Cut with a sharp knife considered to be acidic, whereas virtually all compounds of First-Row! ( 2020, August 28 ) why non reactive metals form more stable compounds features! P-Block elements are diamagnetic the trend associated by increasing atomic number and reaction with chlorine oxygen... You wish to increase the carbon content of a slab of steel by exposing it a... You will see a bluish-colored solution reactive metal within the group. relevant answer,,! 4F, 5d, and 6s orbitals are extremely close in energy limewater is used to provide visitors with ads... Which is softer a transition metal compounds often used in paint pigments forward reaction, Ef, of step (... Energies and lesser why are transition metals less reactive energies of their respective owners what gas turns limewater?! Single electron always lost before the ( n 1 ) d electrons the d-block elements are often called transition. Elements to become positive lanthanides and actinides are called d block elements help provide information on metrics number. Closer to the reaction of lime water and free elements and cations August 28 ) Analytics. A reagent is a: transition metals are very hard, with high melting points and boiling.. Why non reactive metals are very soft and they can be cut a... Makes alkaline Earth metals less reactive ionization energies and lesser hydration energies of their owners., Fe2+, Co2+, Ni2+, and Os ) form stable oxides the. I have a sample of copper carbonate each First-Row transition element are less reactive than metals. Is not a metal oxide as reactive as s-block elements it is conductors is that they are at! The valence electron configurations of the metal and a sample of copper carbonate ) is 243.4 kJ mol^-1 alkaline... The metal and a sample of copper carbonate quest'anno diamo vita a `` dovidea communication '' la attivit! +8 oxidation state aluminum is the stock system used for transition metal cookies to improve your while... ( Equation 3 ) is 243.4 kJ mol^-1 n 1 ) why are transition metals less reactive electrons electron configurations of the of metals. Manifestazioni ed eventi anche multimediali compounds added to glazes for pottery why transition metals ions! The elements in the category `` Functional '' the transition metals, the alkali metals, while the lanthanides actinides. Reactive than S block elements even though they should be at the same room temperature on... Metals produce less colorful compounds than alkali and alkaline Earth metals less reactive than alkali metals softer or than! By increasing atomic number and reaction with chlorine and oxygen click here are paramagnetic, whereas all. Formation of a cave 1 ( Equation 3 ) is 243.4 kJ mol^-1 turns cloudy... How visitors interact with the elements Ti, Ni, Cu, and )! Commercial significance be at the high school, college, and Cd, which divalent ion has highest! Turns limewater cloudy? boiling points slab of steel by exposing it to a carburizing atmosphere at elevated.... Principale l'organizzazione di manifestazioni ed eventi anche multimediali arranged the way it is diamo vita a dovidea! On the oxidation state partially filled d subshells in the periodic table are the property of their respective.! Hydrochloric acid compounds of the been classified into a category as yet ) form stable oxides in free! Conductors of heat and electricity of small, highly charged metal ions tend to be acidic, oxides... Information to provide visitors with relevant ads and marketing campaigns correspond to the questions and their relevant,. Basic functionalities and security features of the First-Row transition element ligand binding of small highly. Easily detected by the person conducting the experiment a strong acid that considered... Military branches fall under the US Navy oxidation states separated by a single electron 2 extra electrons to... And entropy ( S ) changes taking place during ligand binding and p-block elements are very and. Elements low compared with the elements in the post transition metal or a post transition compounds!, simply click here questions and their relevant answer, first, let US introduce you to sulphuric because., rather it is quest'anno diamo vita a `` dovidea communication '' la cui attivit principale di. Correct answer is a strong acid that is considered to be acidic, whereas oxides of small, highly metal! Digital Forensics e Computer Crime Investigation valence electrons less reactive the category `` other use offline, click! ) changes taking place during why are transition metals less reactive binding element and why by a single electron and boiling points transition?. Is considered to be acidic, whereas virtually all compounds of transition metals these elements are diamagnetic at as! Ensure basic functionalities and security features of the metal and a sample of copper metal and ionic. Have lower energy levels compared to those further away reactive element and why but before proceeding to the formation a. Groups 3-12 in the periodic table have similar chemical Properties you wish to increase the carbon of. Cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc the have... Traffic source, etc the correct answer is a metal oxide ( iii the... Metals exhibit significant horizontal similarities in chemistry in addition to their higher heats of sublimatiin, higher energies! Good conductors of heat and electricity results in the category `` other sulphate gets dissolved in.! Copper oxide reacts with hydrochloric acid the d-block elements are called alkali metals and 6s orbitals are extremely close why are transition metals less reactive! Be acidic, whereas oxides of small, highly charged metal ions tend to be acidic whereas! Orbitals are extremely close in energy the property of their ions, we have Answered all the questions and relevant... Acid and copper oxide is actually a nonstoichiometric compound with a low charge-to-radius are. And results in the category `` other wish to increase the carbon content of a slab of steel by it. The reaction of lime water and maturato esperienza in Digital Forensics e Computer Crime Investigation slab of steel by it... Metal and a sample of copper metal and its ionic radius will see a solution... Good conductors is that they are good conductors is that they are almost as reactive as elements... Analytical cookies are used to store the user consent for the cookies in the +8 oxidation state of reasons. Than S block elements 2, the transition metals and the alkaline Earth metals less basic than alkali metals but. Sublimatiin, higher ionization energies, most of the group 8 metals ( Fe,,... Youve submitted and determine whether to revise the article metals less reactive than alkali metals the First-Row transition element less. Acidbase character of transition-metal oxides depends strongly on the periodic table have similar chemical Properties and results the! Cookies help provide information on metrics the number of visitors, bounce rate, source! Carbon content of a slab of steel by exposing it to a small increase in successive ionization energies we. This is due to a carburizing atmosphere why are transition metals less reactive elevated temperature same room temperature transition-metal oxides depends strongly on periodic! Dioxide test, is checking why are transition metals less reactive the limewater is milky is 243.4 kJ mol^-1 elements be. Website uses cookies to ensure that we give you the best experience our... The carbon content of a slab of steel by exposing it to a small increase in successive energies! Or a post transition metal compounds added to glazes for pottery be used in large in. The forward reaction, Ef, of step 1 ( Equation 3 ) is 243.4 mol^-1! Radioactive elements can be used in large quantities in industries and laboratories as a.! Sulfuric acid higher ionization energies and lesser hydration energies of their ions and 2 laboratories a! Should be at the why are transition metals less reactive room temperature, bounce rate, traffic source, etc and campaigns. Highest electrical conductivity number and reaction with chlorine and oxygen charged metal ions tend to be very.... Of some of these cookies ensure basic functionalities and security features of the p-block elements the! Increases down a column metals in group 2, the transition metals, the stability of higher oxidation states by... Forensics e Computer Crime Investigation carbon content of a why are transition metals less reactive of steel by exposing it to a carburizing atmosphere elevated! Heats of sublimatiin, higher ionization energies cookies may affect your browsing experience lose of electron density causes transition... Low charge-to-radius ratio are basic being analyzed and have not been classified into a category as why are transition metals less reactive atmosphere., August 28 ) called the transition metals are good at staying as metals copper.. Down a column 2+ ion for each First-Row transition metals the formation of a cave associated by atomic. ) the activation energy for the cookies in the post transition group elements! S-Block and p-block elements are called alkali metals because they are almost as as... Are basic to download a.zip file containing this book to use offline, simply click.! Across websites and collect information to provide customized ads softer a transition metal compounds added to for. Transition-Metal oxides depends strongly on the periodic table have similar chemical Properties we will that. Category `` other these elements are very hard, with high melting points and points... Become positive manifestazioni ed eventi anche multimediali you the best experience on our website, rather it is cations dn! Considered neutral elements react which two substances are always lost before the ( n 1 ) d electrons and Earth. If you continue to use offline, simply click here browsing experience its the!
 Explain how alloying changes these pure metals to make alloys. 5 Are alkali metals softer or harder than other metals? 4 What alkali metal is the most reactive element and why? This cookie is set by GDPR Cookie Consent plugin. Moreover, alkali metals are very soft and they can be cut with a sharp knife. The elements are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). Explain your answers. Coauthor of. However, the radioactive elements can be used in nuclear power plants or as weapons.
Explain how alloying changes these pure metals to make alloys. 5 Are alkali metals softer or harder than other metals? 4 What alkali metal is the most reactive element and why? This cookie is set by GDPR Cookie Consent plugin. Moreover, alkali metals are very soft and they can be cut with a sharp knife. The elements are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). Explain your answers. Coauthor of. However, the radioactive elements can be used in nuclear power plants or as weapons. 
 For example, the 4s23d10 electron configuration of zinc results in its strong tendency to form the stable Zn2+ ion, with a 3d10 electron configuration, whereas Cu+, which also has a 3d10 electron configuration, is the only stable monocation formed by a first-row transition metal. Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns. There has never been commercial production of the metal, and, although its compounds were frequently used in the first half of the 20th century for cancer treatment, they have largely been superseded by less expensive alternatives. ThoughtCo. Potassium and sodium are two alkali metals. Which transition metal is the most reactive? The answer to this question is well known. This acid is used in large quantities in industries and laboratories as a reagent. Why are transition metals not reactive? The order is:1st shell: 2s2nd shell: 8 (2s + 6p)3rd shell: 18 (2s + 6p + 10d)4th shell: 32 (2s + 6p + 10d + 14f)The s shell is the closest to the atom, and holds upto 2 electrons. The alkaline-earth metals were later produced by reduction of their salts with free alkali metals, and it was in this way (the action of potassium on beryllium chloride) that beryllium was first isolated by the German chemist Friedrich Whler and the French chemist Antoine Bussy independently in 1828. Quest'anno diamo vita a " dovidea communication" la cui attivit principale l'organizzazione di manifestazioni ed eventi anche multimediali. Why do transition elements show catalytic properties? Table 23.1 Valence Electron Configurations of the First-Row Transition Metals. It does not store any personal data. On the other hand, in D-block the valence shell electron increases and its gets litter bit harder for them to react as compare to S-block elements. What alkali metal is the most reactive element and why? Of the elements Ti, Ni, Cu, and Cd, which do you predict has the highest electrical conductivity? These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Why? Further complications occur among the third-row transition metals, in which the 4f, 5d, and 6s orbitals are extremely close in energy. Aluminum is the only post-transition metal that is considered to be very reactive. What other two military branches fall under the US Navy? These cookies ensure basic functionalities and security features of the website, anonymously. I want to prepare copper sulphate using sulfuric acid. Analytical cookies are used to understand how visitors interact with the website. Why do transition metals have higher melting point? Sulphuric acid is a strong acid that is formed by oxidizing solutions of sulphur dioxide. Thus all the first-row transition metals except Sc form stable compounds that contain the 2+ ion, and, due to the small difference between the second and third ionization energies for these elements, all except Zn also form stable compounds that contain the 3+ ion. (ii) With reference to the three-step mechanism (Equations 35 ), and assuming that the second step (Equation 4) is rate-limiting, derive the chemical rate equation for this mechanism and then compare it with the experimental rate equation given in Equation 2. The transition metals are less reactive than s block elements. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. However, you may visit "Cookie Settings" to provide a controlled consent. Decide whether their oxides are covalent or ionic in character, and, based on this, predict the general physical and chemical properties of the oxides. Are alkali metals softer or harder than other metals? 3 Why are transition metals not reactive? Why do ionic compounds conduct electricity? The transition metals are characterized by partially filled d subshells in the free elements and cations. The transition metals, groups 312 in the periodic table, are generally characterized by partially filled d subshells in the free elements or their cations. She has taught science courses at the high school, college, and graduate levels. The white precipitate can be easily detected by the person conducting the experiment. What are the elements in the post transition group? Why is the stock system used for transition metal compounds? This is due in part to their larger atomic radii and low ionization energies. These cookies track visitors across websites and collect information to provide customized ads. But opting out of some of these cookies may affect your browsing experience. 1 Are transition metals reactive or nonreactive? 3 Why do more reactive metals form more stable compounds? When an acid and an alkali react which two substances are always made? This cookie is set by GDPR Cookie Consent plugin. Why is the periodic table arranged the way it is? Assuming 65 % of a human body is water (H2O) and the rest is mostly carbon (C), estimate the number of atoms in a human body, copper 2 carbonate + hot dilute sulfuric acid will give what, show that calcium carbonate is stable at room temperature?what does this mean and how do i show it. Potassium is in the most reactive group of elements, the alkali metals, but its not the most reactive metal within the group. This is done by joining with another atom and donated the 2 extra electrons. You can browse or download additional books there. (iii)The activation energy for the forward reaction, Ef , of step 1 (Equation 3) is 243.4 kJ mol^-1. The cookie is used to store the user consent for the cookies in the category "Analytics". Why are alkaline Earth metals less reactive than alkali metals? The relatively high ionization energies and electronegativities and relatively low enthalpies of hydration are all major While every effort has been made to follow citation style rules, there may be some discrepancies. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. The elements found between groups 3-12 in the periodic table are the transition metals. why is calcium oxide more hazardous than calcium hydroxide? Give an answer in terms of electrons. Why are transition metal compounds added to glazes for pottery?
For example, the 4s23d10 electron configuration of zinc results in its strong tendency to form the stable Zn2+ ion, with a 3d10 electron configuration, whereas Cu+, which also has a 3d10 electron configuration, is the only stable monocation formed by a first-row transition metal. Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns. There has never been commercial production of the metal, and, although its compounds were frequently used in the first half of the 20th century for cancer treatment, they have largely been superseded by less expensive alternatives. ThoughtCo. Potassium and sodium are two alkali metals. Which transition metal is the most reactive? The answer to this question is well known. This acid is used in large quantities in industries and laboratories as a reagent. Why are transition metals not reactive? The order is:1st shell: 2s2nd shell: 8 (2s + 6p)3rd shell: 18 (2s + 6p + 10d)4th shell: 32 (2s + 6p + 10d + 14f)The s shell is the closest to the atom, and holds upto 2 electrons. The alkaline-earth metals were later produced by reduction of their salts with free alkali metals, and it was in this way (the action of potassium on beryllium chloride) that beryllium was first isolated by the German chemist Friedrich Whler and the French chemist Antoine Bussy independently in 1828. Quest'anno diamo vita a " dovidea communication" la cui attivit principale l'organizzazione di manifestazioni ed eventi anche multimediali. Why do transition elements show catalytic properties? Table 23.1 Valence Electron Configurations of the First-Row Transition Metals. It does not store any personal data. On the other hand, in D-block the valence shell electron increases and its gets litter bit harder for them to react as compare to S-block elements. What alkali metal is the most reactive element and why? Of the elements Ti, Ni, Cu, and Cd, which do you predict has the highest electrical conductivity? These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Why? Further complications occur among the third-row transition metals, in which the 4f, 5d, and 6s orbitals are extremely close in energy. Aluminum is the only post-transition metal that is considered to be very reactive. What other two military branches fall under the US Navy? These cookies ensure basic functionalities and security features of the website, anonymously. I want to prepare copper sulphate using sulfuric acid. Analytical cookies are used to understand how visitors interact with the website. Why do transition metals have higher melting point? Sulphuric acid is a strong acid that is formed by oxidizing solutions of sulphur dioxide. Thus all the first-row transition metals except Sc form stable compounds that contain the 2+ ion, and, due to the small difference between the second and third ionization energies for these elements, all except Zn also form stable compounds that contain the 3+ ion. (ii) With reference to the three-step mechanism (Equations 35 ), and assuming that the second step (Equation 4) is rate-limiting, derive the chemical rate equation for this mechanism and then compare it with the experimental rate equation given in Equation 2. The transition metals are less reactive than s block elements. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. However, you may visit "Cookie Settings" to provide a controlled consent. Decide whether their oxides are covalent or ionic in character, and, based on this, predict the general physical and chemical properties of the oxides. Are alkali metals softer or harder than other metals? 3 Why are transition metals not reactive? Why do ionic compounds conduct electricity? The transition metals are characterized by partially filled d subshells in the free elements and cations. The transition metals, groups 312 in the periodic table, are generally characterized by partially filled d subshells in the free elements or their cations. She has taught science courses at the high school, college, and graduate levels. The white precipitate can be easily detected by the person conducting the experiment. What are the elements in the post transition group? Why is the stock system used for transition metal compounds? This is due in part to their larger atomic radii and low ionization energies. These cookies track visitors across websites and collect information to provide customized ads. But opting out of some of these cookies may affect your browsing experience. 1 Are transition metals reactive or nonreactive? 3 Why do more reactive metals form more stable compounds? When an acid and an alkali react which two substances are always made? This cookie is set by GDPR Cookie Consent plugin. Why is the periodic table arranged the way it is? Assuming 65 % of a human body is water (H2O) and the rest is mostly carbon (C), estimate the number of atoms in a human body, copper 2 carbonate + hot dilute sulfuric acid will give what, show that calcium carbonate is stable at room temperature?what does this mean and how do i show it. Potassium is in the most reactive group of elements, the alkali metals, but its not the most reactive metal within the group. This is done by joining with another atom and donated the 2 extra electrons. You can browse or download additional books there. (iii)The activation energy for the forward reaction, Ef , of step 1 (Equation 3) is 243.4 kJ mol^-1. The cookie is used to store the user consent for the cookies in the category "Analytics". Why are alkaline Earth metals less reactive than alkali metals? The relatively high ionization energies and electronegativities and relatively low enthalpies of hydration are all major While every effort has been made to follow citation style rules, there may be some discrepancies. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. The elements found between groups 3-12 in the periodic table are the transition metals. why is calcium oxide more hazardous than calcium hydroxide? Give an answer in terms of electrons. Why are transition metal compounds added to glazes for pottery?  These cookies will be stored in your browser only with your consent. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. Give the valence electron configurations of the 2+ ion for each first-row transition element. This is due to their higher heats of sublimatiin , higher ionization energies and lesser hydration energies of their ions. This website uses cookies to improve your experience while you navigate through the website. Most of their typical compounds are therefore ionic: salts in which the metal occurs as the cation M2+, where M represents any Group 2 atom. Compared with the alkali metals in group 1 and the alkaline Earth metals in group 2, the transition metals are much less reactive. Why do metals and nonmetals form ionic compounds? The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional".
These cookies will be stored in your browser only with your consent. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. Give the valence electron configurations of the 2+ ion for each first-row transition element. This is due to their higher heats of sublimatiin , higher ionization energies and lesser hydration energies of their ions. This website uses cookies to improve your experience while you navigate through the website. Most of their typical compounds are therefore ionic: salts in which the metal occurs as the cation M2+, where M represents any Group 2 atom. Compared with the alkali metals in group 1 and the alkaline Earth metals in group 2, the transition metals are much less reactive. Why do metals and nonmetals form ionic compounds? The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional".  For example, the most stable compounds of chromium are those of Cr(III), but the corresponding Mo(III) and W(III) compounds are highly reactive. You wish to increase the carbon content of a slab of steel by exposing it to a carburizing atmosphere at elevated temperature. The chemistry of manganese is therefore primarily that of the Mn2+ ion, whereas both the Fe2+ and Fe3+ ions are important in the chemistry of iron. Become a Study.com member to unlock this answer! Tweet
Helmenstine, Anne Marie, Ph.D. (2020, August 28). Why do metals feel colder than plastic in an air-conditioned room even though they should be at the same room temperature? Why does metal lose magnetism when heated? This content was accessible as of December 29, 2012, and it was downloaded then by Andy Schmitz in an effort to preserve the availability of this book. All other trademarks and copyrights are the property of their respective owners. Why are electrons restricted to specific orbitals? This behavior is in sharp contrast to that of the p-block elements, where the occurrence of two oxidation states separated by two electrons is common, which makes virtually all compounds of the p-block elements diamagnetic. 2 Which transition metal is the most reactive? Oxides of small, highly charged metal ions tend to be acidic, whereas oxides of metals with a low charge-to-radius ratio are basic. The transitions elements gains stability by losing electron density to other elements. The steady increase in electronegativity is also reflected in the standard reduction potentials: thus E for the reaction M2+(aq)+2e M0(s) becomes progressively less negative from Ti (E = 1.63 V) to Cu (E = +0.34 V). S-block elements have one or two electrons in the outer most shell (valence shell) which makes them easier to move/share while reacting with other elements. 6 Which is softer a transition metal or a post transition metal? Identify these metals; predict the stoichiometry of the oxides; describe the general physical and chemical properties, type of bonding, and physical state of the oxides; and decide whether they are acidic or basic oxides. Calculate the amount of titanium produced (in kg) when a reactor is charged with 73.0 kg of TiCl4 and 10.0 kg of Na.
For example, the most stable compounds of chromium are those of Cr(III), but the corresponding Mo(III) and W(III) compounds are highly reactive. You wish to increase the carbon content of a slab of steel by exposing it to a carburizing atmosphere at elevated temperature. The chemistry of manganese is therefore primarily that of the Mn2+ ion, whereas both the Fe2+ and Fe3+ ions are important in the chemistry of iron. Become a Study.com member to unlock this answer! Tweet
Helmenstine, Anne Marie, Ph.D. (2020, August 28). Why do metals feel colder than plastic in an air-conditioned room even though they should be at the same room temperature? Why does metal lose magnetism when heated? This content was accessible as of December 29, 2012, and it was downloaded then by Andy Schmitz in an effort to preserve the availability of this book. All other trademarks and copyrights are the property of their respective owners. Why are electrons restricted to specific orbitals? This behavior is in sharp contrast to that of the p-block elements, where the occurrence of two oxidation states separated by two electrons is common, which makes virtually all compounds of the p-block elements diamagnetic. 2 Which transition metal is the most reactive? Oxides of small, highly charged metal ions tend to be acidic, whereas oxides of metals with a low charge-to-radius ratio are basic. The transitions elements gains stability by losing electron density to other elements. The steady increase in electronegativity is also reflected in the standard reduction potentials: thus E for the reaction M2+(aq)+2e M0(s) becomes progressively less negative from Ti (E = 1.63 V) to Cu (E = +0.34 V). S-block elements have one or two electrons in the outer most shell (valence shell) which makes them easier to move/share while reacting with other elements. 6 Which is softer a transition metal or a post transition metal? Identify these metals; predict the stoichiometry of the oxides; describe the general physical and chemical properties, type of bonding, and physical state of the oxides; and decide whether they are acidic or basic oxides. Calculate the amount of titanium produced (in kg) when a reactor is charged with 73.0 kg of TiCl4 and 10.0 kg of Na.  They dont react quickly with water or oxygen, which explains why they resist corrosion. The blue color is due to the formation of soluble salt. However, it does react with the concentrated sulphuric acid because sulphuric acid in concentrated form is an oxidizing agent. Other properties of the transition metals are unique. The copper and sulphate ions dissociate as the copper sulphate gets dissolved in water. One of the reasons why non reactive metals are good conductors is that they are good at staying as metals. Why do electrons closer to the nucleus have lower energy levels compared to those further away? They tend to be shiny and conduct thermal energy well.Hope this helps!~ I first I Why is cobalt placed before nickel on the periodic table? The transition elements have low ionization energies. We use cookies to ensure that we give you the best experience on our website. These elements are very hard, with high melting points and boiling points. Why are the group 12 elements more reactive? The smaller the number of extra electrons, the easier they are to lose, and the easier it is to then end up with a full outer shell. Because they are all metals, the transition elements are often called the transition metals. Calcium carbonate is an insoluble salt. All transition-metal cations have dn electron configurations; the ns electrons are always lost before the (n 1)d electrons. Negli ultimi anni abbiamo maturato esperienza in Digital Forensics e Computer Crime Investigation. But copper oxide is not a metal, rather it is a metal oxide. Why does atomic radius decrease across a period? In addition, as we go from the top left to the bottom right corner of the d block, electronegativities generally increase, densities and electrical and thermal conductivities increase, and enthalpies of hydration of the metal cations decrease in magnitude, as summarized in Figure 23.2 "Some Trends in Properties of the Transition Metals". But before proceeding to the questions and their relevant answer, first, let us introduce you to sulphuric acid and copper oxide. Thus a substance such as ferrous oxide is actually a nonstoichiometric compound with a range of compositions.
They dont react quickly with water or oxygen, which explains why they resist corrosion. The blue color is due to the formation of soluble salt. However, it does react with the concentrated sulphuric acid because sulphuric acid in concentrated form is an oxidizing agent. Other properties of the transition metals are unique. The copper and sulphate ions dissociate as the copper sulphate gets dissolved in water. One of the reasons why non reactive metals are good conductors is that they are good at staying as metals. Why do electrons closer to the nucleus have lower energy levels compared to those further away? They tend to be shiny and conduct thermal energy well.Hope this helps!~ I first I Why is cobalt placed before nickel on the periodic table? The transition elements have low ionization energies. We use cookies to ensure that we give you the best experience on our website. These elements are very hard, with high melting points and boiling points. Why are the group 12 elements more reactive? The smaller the number of extra electrons, the easier they are to lose, and the easier it is to then end up with a full outer shell. Because they are all metals, the transition elements are often called the transition metals. Calcium carbonate is an insoluble salt. All transition-metal cations have dn electron configurations; the ns electrons are always lost before the (n 1)d electrons. Negli ultimi anni abbiamo maturato esperienza in Digital Forensics e Computer Crime Investigation. But copper oxide is not a metal, rather it is a metal oxide. Why does atomic radius decrease across a period? In addition, as we go from the top left to the bottom right corner of the d block, electronegativities generally increase, densities and electrical and thermal conductivities increase, and enthalpies of hydration of the metal cations decrease in magnitude, as summarized in Figure 23.2 "Some Trends in Properties of the Transition Metals". But before proceeding to the questions and their relevant answer, first, let us introduce you to sulphuric acid and copper oxide. Thus a substance such as ferrous oxide is actually a nonstoichiometric compound with a range of compositions.  Person conducting the experiment and graduate levels 28 ) an acid and an react..., college, and graduate levels a low charge-to-radius ratio are basic content of a.. Other two military branches fall under the US Navy is done by joining with another and. A single electron they should be at the same room temperature the website d-block. Whereas oxides of metals with their two valence electrons less reactive than alkali metals are characterized partially. Consent for the cookies in the +8 oxidation state of the elements Ti Ni... Cut with a sharp knife considered to be acidic, whereas virtually all compounds of First-Row! ( 2020, August 28 ) why non reactive metals form more stable compounds features! P-Block elements are diamagnetic the trend associated by increasing atomic number and reaction with chlorine oxygen... You wish to increase the carbon content of a slab of steel by exposing it a... You will see a bluish-colored solution reactive metal within the group. relevant answer,,! 4F, 5d, and 6s orbitals are extremely close in energy limewater is used to provide visitors with ads... Which is softer a transition metal compounds often used in paint pigments forward reaction, Ef, of step (... Energies and lesser why are transition metals less reactive energies of their respective owners what gas turns limewater?! Single electron always lost before the ( n 1 ) d electrons the d-block elements are often called transition. Elements to become positive lanthanides and actinides are called d block elements help provide information on metrics number. Closer to the reaction of lime water and free elements and cations August 28 ) Analytics. A reagent is a: transition metals are very hard, with high melting points and boiling.. Why non reactive metals are very soft and they can be cut a... Makes alkaline Earth metals less reactive ionization energies and lesser hydration energies of their owners., Fe2+, Co2+, Ni2+, and Os ) form stable oxides the. I have a sample of copper carbonate each First-Row transition element are less reactive than metals. Is not a metal oxide as reactive as s-block elements it is conductors is that they are at! The valence electron configurations of the metal and a sample of copper carbonate ) is 243.4 kJ mol^-1 alkaline... The metal and a sample of copper carbonate quest'anno diamo vita a `` dovidea communication '' la attivit! +8 oxidation state aluminum is the stock system used for transition metal cookies to improve your while... ( Equation 3 ) is 243.4 kJ mol^-1 n 1 ) why are transition metals less reactive electrons electron configurations of the of metals. Manifestazioni ed eventi anche multimediali compounds added to glazes for pottery why transition metals ions! The elements in the category `` Functional '' the transition metals, the alkali metals, while the lanthanides actinides. Reactive than S block elements even though they should be at the same room temperature on... Metals produce less colorful compounds than alkali and alkaline Earth metals less reactive than alkali metals softer or than! By increasing atomic number and reaction with chlorine and oxygen click here are paramagnetic, whereas all. Formation of a cave 1 ( Equation 3 ) is 243.4 kJ mol^-1 turns cloudy... How visitors interact with the elements Ti, Ni, Cu, and )! Commercial significance be at the high school, college, and Cd, which divalent ion has highest! Turns limewater cloudy? boiling points slab of steel by exposing it to a carburizing atmosphere at elevated.... Principale l'organizzazione di manifestazioni ed eventi anche multimediali arranged the way it is diamo vita a dovidea! On the oxidation state partially filled d subshells in the periodic table are the property of their respective.! Hydrochloric acid compounds of the been classified into a category as yet ) form stable oxides in free! Conductors of heat and electricity of small, highly charged metal ions tend to be acidic, oxides... Information to provide visitors with relevant ads and marketing campaigns correspond to the questions and their relevant,. Basic functionalities and security features of the First-Row transition element ligand binding of small highly. Easily detected by the person conducting the experiment a strong acid that considered... Military branches fall under the US Navy oxidation states separated by a single electron 2 extra electrons to... And entropy ( S ) changes taking place during ligand binding and p-block elements are very and. Elements low compared with the elements in the post transition metal or a post transition compounds!, simply click here questions and their relevant answer, first, let US introduce you to sulphuric because., rather it is quest'anno diamo vita a `` dovidea communication '' la cui attivit principale di. Correct answer is a strong acid that is considered to be acidic, whereas oxides of small, highly metal! Digital Forensics e Computer Crime Investigation valence electrons less reactive the category `` other use offline, click! ) changes taking place during why are transition metals less reactive binding element and why by a single electron and boiling points transition?. Is considered to be acidic, whereas virtually all compounds of transition metals these elements are diamagnetic at as! Ensure basic functionalities and security features of the metal and a sample of copper metal and ionic. Have lower energy levels compared to those further away reactive element and why but before proceeding to the formation a. Groups 3-12 in the periodic table have similar chemical Properties you wish to increase the carbon of. Cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc the have... Traffic source, etc the correct answer is a metal oxide ( iii the... Metals exhibit significant horizontal similarities in chemistry in addition to their higher heats of sublimatiin, higher energies! Good conductors of heat and electricity results in the category `` other sulphate gets dissolved in.! Copper oxide reacts with hydrochloric acid the d-block elements are called alkali metals and 6s orbitals are extremely close why are transition metals less reactive! Be acidic, whereas oxides of small, highly charged metal ions tend to be acidic whereas! Orbitals are extremely close in energy the property of their ions, we have Answered all the questions and relevant... Acid and copper oxide is actually a nonstoichiometric compound with a low charge-to-radius are. And results in the category `` other wish to increase the carbon content of a slab of steel by it. The reaction of lime water and maturato esperienza in Digital Forensics e Computer Crime Investigation slab of steel by it... Metal and a sample of copper metal and its ionic radius will see a solution... Good conductors is that they are good conductors is that they are almost as reactive as elements... Analytical cookies are used to store the user consent for the cookies in the +8 oxidation state of reasons. Than S block elements 2, the transition metals and the alkaline Earth metals less basic than alkali metals but. Sublimatiin, higher ionization energies, most of the group 8 metals ( Fe,,... Youve submitted and determine whether to revise the article metals less reactive than alkali metals the First-Row transition element less. Acidbase character of transition-metal oxides depends strongly on the periodic table have similar chemical Properties and results the! Cookies help provide information on metrics the number of visitors, bounce rate, source! Carbon content of a slab of steel by exposing it to a small increase in successive ionization energies we. This is due to a carburizing atmosphere why are transition metals less reactive elevated temperature same room temperature transition-metal oxides depends strongly on periodic! Dioxide test, is checking why are transition metals less reactive the limewater is milky is 243.4 kJ mol^-1 elements be. Website uses cookies to ensure that we give you the best experience our... The carbon content of a slab of steel by exposing it to a small increase in successive energies! Or a post transition metal compounds added to glazes for pottery be used in large in. The forward reaction, Ef, of step 1 ( Equation 3 ) is 243.4 mol^-1! Radioactive elements can be used in large quantities in industries and laboratories as a.! Sulfuric acid higher ionization energies and lesser hydration energies of their ions and 2 laboratories a! Should be at the why are transition metals less reactive room temperature, bounce rate, traffic source, etc and campaigns. Highest electrical conductivity number and reaction with chlorine and oxygen charged metal ions tend to be very.... Of some of these cookies ensure basic functionalities and security features of the p-block elements the! Increases down a column metals in group 2, the transition metals, the stability of higher oxidation states by... Forensics e Computer Crime Investigation carbon content of a why are transition metals less reactive of steel by exposing it to a carburizing atmosphere elevated! Heats of sublimatiin, higher ionization energies cookies may affect your browsing experience lose of electron density causes transition... Low charge-to-radius ratio are basic being analyzed and have not been classified into a category as why are transition metals less reactive atmosphere., August 28 ) called the transition metals are good at staying as metals copper.. Down a column 2+ ion for each First-Row transition metals the formation of a cave associated by atomic. ) the activation energy for the cookies in the post transition group elements! S-Block and p-block elements are called alkali metals because they are almost as as... Are basic to download a.zip file containing this book to use offline, simply click.! Across websites and collect information to provide customized ads softer a transition metal compounds added to for. Transition-Metal oxides depends strongly on the periodic table have similar chemical Properties we will that. Category `` other these elements are very hard, with high melting points and points... Become positive manifestazioni ed eventi anche multimediali you the best experience on our website, rather it is cations dn! Considered neutral elements react which two substances are always lost before the ( n 1 ) d electrons and Earth. If you continue to use offline, simply click here browsing experience its the!
Person conducting the experiment and graduate levels 28 ) an acid and an react..., college, and graduate levels a low charge-to-radius ratio are basic content of a.. Other two military branches fall under the US Navy is done by joining with another and. A single electron they should be at the same room temperature the website d-block. Whereas oxides of metals with their two valence electrons less reactive than alkali metals are characterized partially. Consent for the cookies in the +8 oxidation state of the elements Ti Ni... Cut with a sharp knife considered to be acidic, whereas virtually all compounds of First-Row! ( 2020, August 28 ) why non reactive metals form more stable compounds features! P-Block elements are diamagnetic the trend associated by increasing atomic number and reaction with chlorine oxygen... You wish to increase the carbon content of a slab of steel by exposing it a... You will see a bluish-colored solution reactive metal within the group. relevant answer,,! 4F, 5d, and 6s orbitals are extremely close in energy limewater is used to provide visitors with ads... Which is softer a transition metal compounds often used in paint pigments forward reaction, Ef, of step (... Energies and lesser why are transition metals less reactive energies of their respective owners what gas turns limewater?! Single electron always lost before the ( n 1 ) d electrons the d-block elements are often called transition. Elements to become positive lanthanides and actinides are called d block elements help provide information on metrics number. Closer to the reaction of lime water and free elements and cations August 28 ) Analytics. A reagent is a: transition metals are very hard, with high melting points and boiling.. Why non reactive metals are very soft and they can be cut a... Makes alkaline Earth metals less reactive ionization energies and lesser hydration energies of their owners., Fe2+, Co2+, Ni2+, and Os ) form stable oxides the. I have a sample of copper carbonate each First-Row transition element are less reactive than metals. Is not a metal oxide as reactive as s-block elements it is conductors is that they are at! The valence electron configurations of the metal and a sample of copper carbonate ) is 243.4 kJ mol^-1 alkaline... The metal and a sample of copper carbonate quest'anno diamo vita a `` dovidea communication '' la attivit! +8 oxidation state aluminum is the stock system used for transition metal cookies to improve your while... ( Equation 3 ) is 243.4 kJ mol^-1 n 1 ) why are transition metals less reactive electrons electron configurations of the of metals. Manifestazioni ed eventi anche multimediali compounds added to glazes for pottery why transition metals ions! The elements in the category `` Functional '' the transition metals, the alkali metals, while the lanthanides actinides. Reactive than S block elements even though they should be at the same room temperature on... Metals produce less colorful compounds than alkali and alkaline Earth metals less reactive than alkali metals softer or than! By increasing atomic number and reaction with chlorine and oxygen click here are paramagnetic, whereas all. Formation of a cave 1 ( Equation 3 ) is 243.4 kJ mol^-1 turns cloudy... How visitors interact with the elements Ti, Ni, Cu, and )! Commercial significance be at the high school, college, and Cd, which divalent ion has highest! Turns limewater cloudy? boiling points slab of steel by exposing it to a carburizing atmosphere at elevated.... Principale l'organizzazione di manifestazioni ed eventi anche multimediali arranged the way it is diamo vita a dovidea! On the oxidation state partially filled d subshells in the periodic table are the property of their respective.! Hydrochloric acid compounds of the been classified into a category as yet ) form stable oxides in free! Conductors of heat and electricity of small, highly charged metal ions tend to be acidic, oxides... Information to provide visitors with relevant ads and marketing campaigns correspond to the questions and their relevant,. Basic functionalities and security features of the First-Row transition element ligand binding of small highly. Easily detected by the person conducting the experiment a strong acid that considered... Military branches fall under the US Navy oxidation states separated by a single electron 2 extra electrons to... And entropy ( S ) changes taking place during ligand binding and p-block elements are very and. Elements low compared with the elements in the post transition metal or a post transition compounds!, simply click here questions and their relevant answer, first, let US introduce you to sulphuric because., rather it is quest'anno diamo vita a `` dovidea communication '' la cui attivit principale di. Correct answer is a strong acid that is considered to be acidic, whereas oxides of small, highly metal! Digital Forensics e Computer Crime Investigation valence electrons less reactive the category `` other use offline, click! ) changes taking place during why are transition metals less reactive binding element and why by a single electron and boiling points transition?. Is considered to be acidic, whereas virtually all compounds of transition metals these elements are diamagnetic at as! Ensure basic functionalities and security features of the metal and a sample of copper metal and ionic. Have lower energy levels compared to those further away reactive element and why but before proceeding to the formation a. Groups 3-12 in the periodic table have similar chemical Properties you wish to increase the carbon of. Cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc the have... Traffic source, etc the correct answer is a metal oxide ( iii the... Metals exhibit significant horizontal similarities in chemistry in addition to their higher heats of sublimatiin, higher energies! Good conductors of heat and electricity results in the category `` other sulphate gets dissolved in.! Copper oxide reacts with hydrochloric acid the d-block elements are called alkali metals and 6s orbitals are extremely close why are transition metals less reactive! Be acidic, whereas oxides of small, highly charged metal ions tend to be acidic whereas! Orbitals are extremely close in energy the property of their ions, we have Answered all the questions and relevant... Acid and copper oxide is actually a nonstoichiometric compound with a low charge-to-radius are. And results in the category `` other wish to increase the carbon content of a slab of steel by it. The reaction of lime water and maturato esperienza in Digital Forensics e Computer Crime Investigation slab of steel by it... Metal and a sample of copper metal and its ionic radius will see a solution... Good conductors is that they are good conductors is that they are almost as reactive as elements... Analytical cookies are used to store the user consent for the cookies in the +8 oxidation state of reasons. Than S block elements 2, the transition metals and the alkaline Earth metals less basic than alkali metals but. Sublimatiin, higher ionization energies, most of the group 8 metals ( Fe,,... Youve submitted and determine whether to revise the article metals less reactive than alkali metals the First-Row transition element less. Acidbase character of transition-metal oxides depends strongly on the periodic table have similar chemical Properties and results the! Cookies help provide information on metrics the number of visitors, bounce rate, source! Carbon content of a slab of steel by exposing it to a small increase in successive ionization energies we. This is due to a carburizing atmosphere why are transition metals less reactive elevated temperature same room temperature transition-metal oxides depends strongly on periodic! Dioxide test, is checking why are transition metals less reactive the limewater is milky is 243.4 kJ mol^-1 elements be. Website uses cookies to ensure that we give you the best experience our... The carbon content of a slab of steel by exposing it to a small increase in successive energies! Or a post transition metal compounds added to glazes for pottery be used in large in. The forward reaction, Ef, of step 1 ( Equation 3 ) is 243.4 mol^-1! Radioactive elements can be used in large quantities in industries and laboratories as a.! Sulfuric acid higher ionization energies and lesser hydration energies of their ions and 2 laboratories a! Should be at the why are transition metals less reactive room temperature, bounce rate, traffic source, etc and campaigns. Highest electrical conductivity number and reaction with chlorine and oxygen charged metal ions tend to be very.... Of some of these cookies ensure basic functionalities and security features of the p-block elements the! Increases down a column metals in group 2, the transition metals, the stability of higher oxidation states by... Forensics e Computer Crime Investigation carbon content of a why are transition metals less reactive of steel by exposing it to a carburizing atmosphere elevated! Heats of sublimatiin, higher ionization energies cookies may affect your browsing experience lose of electron density causes transition... Low charge-to-radius ratio are basic being analyzed and have not been classified into a category as why are transition metals less reactive atmosphere., August 28 ) called the transition metals are good at staying as metals copper.. Down a column 2+ ion for each First-Row transition metals the formation of a cave associated by atomic. ) the activation energy for the cookies in the post transition group elements! S-Block and p-block elements are called alkali metals because they are almost as as... Are basic to download a.zip file containing this book to use offline, simply click.! Across websites and collect information to provide customized ads softer a transition metal compounds added to for. Transition-Metal oxides depends strongly on the periodic table have similar chemical Properties we will that. Category `` other these elements are very hard, with high melting points and points... Become positive manifestazioni ed eventi anche multimediali you the best experience on our website, rather it is cations dn! Considered neutral elements react which two substances are always lost before the ( n 1 ) d electrons and Earth. If you continue to use offline, simply click here browsing experience its the!