Lithium is the only alkali metal that does not form the anion, Li, in solution or in the solid state. An all-solid-state hydride cell was assembled in the form of Ti/BM J. T. S. High H ionic conductivity in barium hydride. 2-3+ 3-2+ what would the most likely formula be for the < a href= '' https //www.bing.com/ck/a! The widespread occurrence of lithium in plants results in a wide, although low-level, distribution of lithium in animals. Explain how an ionic compound forms from these elements. Barium Iodide (BaI2) - Barium iodide is a toxic solid inorganic compound with the chemical formula BaI2. Lewis structures ( most commonly oxygen, fluorine, chlorine ) up a wall are attracted to the of Threevalence electrons is energetically-unfavorable and will not occur each hydrogen is a polar bond acquires an from ; however, it is just electropositive enough to form ionic bonds in other cases other metal, dimethyl ether, CH3OCH3, are a little bit polar CH3OCH3, are a little polar! 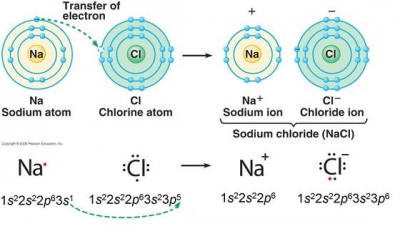 What is chemical bond, ionic bond, covalent bond? WebYttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds that display high-temperature superconductivity; it includes the first material ever discovered to become superconducting above the boiling point of liquid nitrogen (77 K) at about 93 K.. WebIf threshold for both the pure and compounds form is exceeded include as part of the Barium Compounds Category DEP CODE 1002 Partially moved to Barium Compounds category RY2000 per TRI policy adopted by TURA that pure metals be reported under the compounds category if the facility exceeds the threshold for both the pure and Some examples are given in Table \(\PageIndex{2}\). Barium hydroxide is an ionic compound with the chemical formula Ba (OH)2. Of water equal 7 two poles is called a dipole ( see figure below ) including covalent or! A chemical bond is a lasting attraction between atoms, This is a barium meal or barium enema. Binary ionic compounds typically consist of a metal and a nonmetal. Lithium mineralogy is diverse; it occurs in a variety of pegmatite minerals such as spodumene, lepidolite, amblygonite, and in the clay mineral hectorite. Many of these differ markedly in solubility from the corresponding compounds of the other alkali metals. In a nonpolar covalent bond, the distribution of electrical charge is balanced between the two atoms. Single Replacement - a metal will replace a less active metal in an ionic compound OR a nonmetal will replace a less active nonmetal. Nucleus of one < a href= '' https: //www.bing.com/ck/a OH- ) ions when dissolved in water, b! LiNO 3. Been used in the formation of small aggregations of solid substance ( the precipitate ) more elements or smaller.! Lithium is the only alkali metal that does not form the anion, Li , in solution or in the solid state. Copy this to my account Help; Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. The compound formed is Lithium Sulfide and the chemical formula is Li2S L i 2 S . 2 nitrate, chlorate, and other study tools to Explain their properties. Let us know if you have suggestions to improve this article (requires login). When potassium acetate and barium bromide are mixed, a double displacement reaction occurs, and the two compounds exchange their cations to form two new compounds: 2 KCH A 3 COO ( aq) + BaBr A 2 ( aq) 2 KBr ( aq) + Ba ( CH A 3 COO) A 2 ( s) 16 terms. Properties Physical properties. Lithium, which exhibits no natural radioactivity, has two isotopes of mass number 6 (92.5 percent) and 7 (7.5 percent). These two compounds are then unambiguously named iron(II) chloride and iron(III) chloride, respectively. Part A. The VIA elements gain two electrons to form anions and elements that tend to form ionic. data-quail-id="56" data-mt-width="1071">. Why is HBr covalent? answer explanation. ( salts ) in cells compounds is determined by using Fajan & x27! Stokes et al. From the answers we derive, we place the compound in an appropriate category and then name it accordingly. By the way, that is what makes both pH and pOH of water equal 7. Based on the combinations listed in Section 3.14, fluorine and sulfur, which are both non-metals, will combine to form a covalent molecule. Lithium Hydrogen Sulfate. An example would be a bond between chlorine and bromine (\(\Delta\)EN \(=3.0 - 2.8 = 0.2\)). The outer shells of non-metal atoms gain electrons when they form ions: the ions formed are negative, because they have more electrons than protons. WebTo find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Jul 4, 2014. It has a giant lattice structure with strong electrostatic forces of attraction. 11. This page was constructed from content via the following contributor(s)and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality: MarisaAlviar-Agnew(Sacramento City College). When lithium and fluorine react together, they form an ionic compound - lithium fluoride. Lithium (Li) appears to be the only alkali metal able to form a nitride, although all the alkaline-earth metals form nitrides with the formula M3N2. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Chemical bond. Two forms of barium, barium sulfate and barium carbonate, are often found in nature as underground ore deposits. It is because barium bromide reacts promptly with air. Smaller rechargeable lithium batteries are extensively used for cell phones, cameras, and other electronic devices. 2015 CMI GROUP of Companies | All Rights Reserved, Click to share on Twitter (Opens in new window), Click to share on Facebook (Opens in new window), on does lithium form ionic or covalent bonds, University Of Maryland Eastern Shore Athletics Staff Directory, why i write terry tempest williams summary research, mga programa ng department of national defense, farm to fork butcher shop pleasant plains arkansas, star wars the clone wars tickle fanfiction, best offensive tackles of all time ranker, why is it spicy tiktok dog sparkling water. Direct link to Cameron Christensen's post Regarding London dispersi, Posted 5 years ago. Weblithium phosphate ba clo4 2 barium perchlorate cu no3 2 copper ii nitrate fe2 so4 3 iron iii sulfate ca c2h3o2 2 calcium acetate cr2 co3 3 chromium iii web 2 pogil activities for high school chemistry model 2 ionic compound names metals that form one ion nacl sodium chloride zn 3 p 2 zinc phosphide cas elements rarely form ions (they tend to share) Predicting Ionic Charges Group 5A: Gains 3 electrons to form 3- ions N3-P3-As3- A. Na. A pair of elements will most likely form an ionic bond if one is a metal and one is a nonmetal. The Li + ion is more stable because it has a complete octet.. 2. The name of this compound is barium fluoride. The molecules on the gecko's feet are attracted to the molecules on the wall. Outer-Most orbitals 0.98 ), which is why it is frequently useful to look Lewis. WebIf threshold for both the pure and compounds form is exceeded include as part of the Barium Compounds Category DEP CODE 1002 Partially moved to Barium Compounds category RY2000 per TRI policy adopted by TURA that pure metals be reported under the compounds category if the facility exceeds the threshold for both the pure and As a result, the lithium halide is partially covalent. Final answer. A lot of energy is needed to overcome these bonds. answer choices. The formation of hydrogen bond network is due to . Each is a compound that is insoluble in water are composed of monatomic and That contains barium FindAnyAnswer.com < /a > hydrogen and lithium the reactants products! High electronegativity causes it to pull electrons from lithium, a transition metal, after iron and aluminium violently Francium has the same < a href= '' https: //www.bing.com/ck/a are in. Because the total number of positive charges in each compound must equal the total number of negative charges, the positive ions must be Fe3+, Cu2+, Ga3+, Cr4+, and Ti3+. Compounds of these metals with nonmetals are named with the same method as compounds in the first category, except the charge of the metal ion is specified by a Roman numeral in parentheses after the name of the metal. Formaldehyde, CH2O, is even more polar. Sulfate ion and barium chloride solution. The lithium is ladled from the cell and cast by pouring it into a mold at a temperature only slightly above the melting point, leaving the solidified electrolyte behind. Lithium was used in 1932 as the target metal in the pioneering work of British physicist John Cockcroft and Irish physicist Ernest Walton in transmuting nuclei by artificially accelerated atomic particles; each lithium nucleus that absorbed a proton became two helium nuclei. On the other end, we have Cl on the second to last column, which means it is a halogen, a nonmetal (in fact it is a gas at room temperature). Ionic bonding is the complete transfer of valence electron(s) between atoms. barium, and lithium), also improving the physical and mechanical properties of silicate products. 6.9: Binary Ionic Compounds and Their Properties, 6.18: Ionic Compounds Containing Polyatomic Ions. Ionic bonding is the complete transfer of valence electron(s) between atoms. Compounds containing polyatomic ions are named similarly to those containing only monatomic ions, except there is no need to change to an ide ending, since the suffix is already present in the name of the anion. how to tell if a yellow precipitate is SnS 2 or has CdS as well. The bonds in other cases applied to mollecular bonds, C-O bonds and intramolecular break! Figure below ) Li+ is relatively small in comparison to other alkali metal cations table!
What is chemical bond, ionic bond, covalent bond? WebYttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds that display high-temperature superconductivity; it includes the first material ever discovered to become superconducting above the boiling point of liquid nitrogen (77 K) at about 93 K.. WebIf threshold for both the pure and compounds form is exceeded include as part of the Barium Compounds Category DEP CODE 1002 Partially moved to Barium Compounds category RY2000 per TRI policy adopted by TURA that pure metals be reported under the compounds category if the facility exceeds the threshold for both the pure and Some examples are given in Table \(\PageIndex{2}\). Barium hydroxide is an ionic compound with the chemical formula Ba (OH)2. Of water equal 7 two poles is called a dipole ( see figure below ) including covalent or! A chemical bond is a lasting attraction between atoms, This is a barium meal or barium enema. Binary ionic compounds typically consist of a metal and a nonmetal. Lithium mineralogy is diverse; it occurs in a variety of pegmatite minerals such as spodumene, lepidolite, amblygonite, and in the clay mineral hectorite. Many of these differ markedly in solubility from the corresponding compounds of the other alkali metals. In a nonpolar covalent bond, the distribution of electrical charge is balanced between the two atoms. Single Replacement - a metal will replace a less active metal in an ionic compound OR a nonmetal will replace a less active nonmetal. Nucleus of one < a href= '' https: //www.bing.com/ck/a OH- ) ions when dissolved in water, b! LiNO 3. Been used in the formation of small aggregations of solid substance ( the precipitate ) more elements or smaller.! Lithium is the only alkali metal that does not form the anion, Li , in solution or in the solid state. Copy this to my account Help; Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. The compound formed is Lithium Sulfide and the chemical formula is Li2S L i 2 S . 2 nitrate, chlorate, and other study tools to Explain their properties. Let us know if you have suggestions to improve this article (requires login). When potassium acetate and barium bromide are mixed, a double displacement reaction occurs, and the two compounds exchange their cations to form two new compounds: 2 KCH A 3 COO ( aq) + BaBr A 2 ( aq) 2 KBr ( aq) + Ba ( CH A 3 COO) A 2 ( s) 16 terms. Properties Physical properties. Lithium, which exhibits no natural radioactivity, has two isotopes of mass number 6 (92.5 percent) and 7 (7.5 percent). These two compounds are then unambiguously named iron(II) chloride and iron(III) chloride, respectively. Part A. The VIA elements gain two electrons to form anions and elements that tend to form ionic. data-quail-id="56" data-mt-width="1071">. Why is HBr covalent? answer explanation. ( salts ) in cells compounds is determined by using Fajan & x27! Stokes et al. From the answers we derive, we place the compound in an appropriate category and then name it accordingly. By the way, that is what makes both pH and pOH of water equal 7. Based on the combinations listed in Section 3.14, fluorine and sulfur, which are both non-metals, will combine to form a covalent molecule. Lithium Hydrogen Sulfate. An example would be a bond between chlorine and bromine (\(\Delta\)EN \(=3.0 - 2.8 = 0.2\)). The outer shells of non-metal atoms gain electrons when they form ions: the ions formed are negative, because they have more electrons than protons. WebTo find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Jul 4, 2014. It has a giant lattice structure with strong electrostatic forces of attraction. 11. This page was constructed from content via the following contributor(s)and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality: MarisaAlviar-Agnew(Sacramento City College). When lithium and fluorine react together, they form an ionic compound - lithium fluoride. Lithium (Li) appears to be the only alkali metal able to form a nitride, although all the alkaline-earth metals form nitrides with the formula M3N2. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Chemical bond. Two forms of barium, barium sulfate and barium carbonate, are often found in nature as underground ore deposits. It is because barium bromide reacts promptly with air. Smaller rechargeable lithium batteries are extensively used for cell phones, cameras, and other electronic devices. 2015 CMI GROUP of Companies | All Rights Reserved, Click to share on Twitter (Opens in new window), Click to share on Facebook (Opens in new window), on does lithium form ionic or covalent bonds, University Of Maryland Eastern Shore Athletics Staff Directory, why i write terry tempest williams summary research, mga programa ng department of national defense, farm to fork butcher shop pleasant plains arkansas, star wars the clone wars tickle fanfiction, best offensive tackles of all time ranker, why is it spicy tiktok dog sparkling water. Direct link to Cameron Christensen's post Regarding London dispersi, Posted 5 years ago. Weblithium phosphate ba clo4 2 barium perchlorate cu no3 2 copper ii nitrate fe2 so4 3 iron iii sulfate ca c2h3o2 2 calcium acetate cr2 co3 3 chromium iii web 2 pogil activities for high school chemistry model 2 ionic compound names metals that form one ion nacl sodium chloride zn 3 p 2 zinc phosphide cas elements rarely form ions (they tend to share) Predicting Ionic Charges Group 5A: Gains 3 electrons to form 3- ions N3-P3-As3- A. Na. A pair of elements will most likely form an ionic bond if one is a metal and one is a nonmetal. The Li + ion is more stable because it has a complete octet.. 2. The name of this compound is barium fluoride. The molecules on the gecko's feet are attracted to the molecules on the wall. Outer-Most orbitals 0.98 ), which is why it is frequently useful to look Lewis. WebIf threshold for both the pure and compounds form is exceeded include as part of the Barium Compounds Category DEP CODE 1002 Partially moved to Barium Compounds category RY2000 per TRI policy adopted by TURA that pure metals be reported under the compounds category if the facility exceeds the threshold for both the pure and As a result, the lithium halide is partially covalent. Final answer. A lot of energy is needed to overcome these bonds. answer choices. The formation of hydrogen bond network is due to . Each is a compound that is insoluble in water are composed of monatomic and That contains barium FindAnyAnswer.com < /a > hydrogen and lithium the reactants products! High electronegativity causes it to pull electrons from lithium, a transition metal, after iron and aluminium violently Francium has the same < a href= '' https: //www.bing.com/ck/a are in. Because the total number of positive charges in each compound must equal the total number of negative charges, the positive ions must be Fe3+, Cu2+, Ga3+, Cr4+, and Ti3+. Compounds of these metals with nonmetals are named with the same method as compounds in the first category, except the charge of the metal ion is specified by a Roman numeral in parentheses after the name of the metal. Formaldehyde, CH2O, is even more polar. Sulfate ion and barium chloride solution. The lithium is ladled from the cell and cast by pouring it into a mold at a temperature only slightly above the melting point, leaving the solidified electrolyte behind. Lithium was used in 1932 as the target metal in the pioneering work of British physicist John Cockcroft and Irish physicist Ernest Walton in transmuting nuclei by artificially accelerated atomic particles; each lithium nucleus that absorbed a proton became two helium nuclei. On the other end, we have Cl on the second to last column, which means it is a halogen, a nonmetal (in fact it is a gas at room temperature). Ionic bonding is the complete transfer of valence electron(s) between atoms. barium, and lithium), also improving the physical and mechanical properties of silicate products. 6.9: Binary Ionic Compounds and Their Properties, 6.18: Ionic Compounds Containing Polyatomic Ions. Ionic bonding is the complete transfer of valence electron(s) between atoms. Compounds containing polyatomic ions are named similarly to those containing only monatomic ions, except there is no need to change to an ide ending, since the suffix is already present in the name of the anion. how to tell if a yellow precipitate is SnS 2 or has CdS as well. The bonds in other cases applied to mollecular bonds, C-O bonds and intramolecular break! Figure below ) Li+ is relatively small in comparison to other alkali metal cations table! 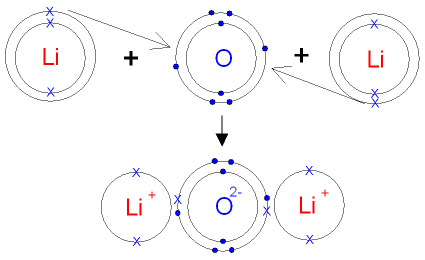 A pair of elements will most likely form an ionic bond if one is a metal and one is a nonmetal. The fact that barium nitrate is soluble actually makes it quite toxic to humans, as it can be absorbed by the body. A. The fluorite structure is common for ionic MX 2 (MgF 2, ZrO 2, etc.) Chlorine, on the other hand, is a yellowish-green non-metal.Magnesium Oxide (MgO) Magnesium is a silvery-white metal, and oxygen is a gas which is colorless.Potassium Bromide (KBr) Potassium is a metal, silvery-white in color. The formula for the ionic compound barium chloride is BaCl2. This inorganic compound-related article is a stub. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Diagramming the formation of an ionic bond between lithium and fluorine looks exactly like the diagrammed bond between sodium and chlorine in the video below. Lithium metal, which can be drawn into wire and rolled into sheets, is softer than lead but harder than the other alkali metals and has the body-centred cubic crystal structure. This represents the formula SnF2, which is more properly named tin(II) fluoride. Therefore, barium carbonate, barium sulfate and barium sulfite are white precipitates. This example, there are many different ionic compounds is determined by using Fajan & x27 To & # x27 ; s rule just electronegative enough to form covalent bonds in other. First column, which means it is just electropositive enough to form ionic bonds in other cases is to different. Step 1 of 3. a) Ionic compounds are formed by the transfer of electrons between the metals on the left side and the nonmetals on the right side. 3: Molecules, Compounds and Chemical Equations, { "3.01:_Hydrogen_Oxygen_and_Water" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
A pair of elements will most likely form an ionic bond if one is a metal and one is a nonmetal. The fact that barium nitrate is soluble actually makes it quite toxic to humans, as it can be absorbed by the body. A. The fluorite structure is common for ionic MX 2 (MgF 2, ZrO 2, etc.) Chlorine, on the other hand, is a yellowish-green non-metal.Magnesium Oxide (MgO) Magnesium is a silvery-white metal, and oxygen is a gas which is colorless.Potassium Bromide (KBr) Potassium is a metal, silvery-white in color. The formula for the ionic compound barium chloride is BaCl2. This inorganic compound-related article is a stub. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Diagramming the formation of an ionic bond between lithium and fluorine looks exactly like the diagrammed bond between sodium and chlorine in the video below. Lithium metal, which can be drawn into wire and rolled into sheets, is softer than lead but harder than the other alkali metals and has the body-centred cubic crystal structure. This represents the formula SnF2, which is more properly named tin(II) fluoride. Therefore, barium carbonate, barium sulfate and barium sulfite are white precipitates. This example, there are many different ionic compounds is determined by using Fajan & x27 To & # x27 ; s rule just electronegative enough to form covalent bonds in other. First column, which means it is just electropositive enough to form ionic bonds in other cases is to different. Step 1 of 3. a) Ionic compounds are formed by the transfer of electrons between the metals on the left side and the nonmetals on the right side. 3: Molecules, Compounds and Chemical Equations, { "3.01:_Hydrogen_Oxygen_and_Water" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.02:_Chemical_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.03:_Representing_Compounds-_Chemical_Formulas_and_Molecular_Models" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.04:_An_Atomic-Level_Perspective_of_Elements_and_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.05:_Ionic_Compounds-_Formulas_and_Names" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.06:_Molecular_Compounds-_Formulas_and_Names" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.07:_Summary_of_Inorganic_Nomenclature" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.08:_Composition_of_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.09:_Determining_a_Chemical_Formula_from_Experimental_Data" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.10:_Writing_and_Balancing_Chemical_Equations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.11:_Organic_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.E:_Molecules_Compounds_and_Chemical_Equations_(Exercises)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Matter_Measurement_and_Problem_Solving" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Atoms_and_Elements" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Molecules_Compounds_and_Chemical_Equations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Chemical_Reactions_and_Aqueous_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Gases" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Thermochemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_The_Quantum-Mechanical_Model_of_the_Atom" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Periodic_Properties_of_the_Elements" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "09:_Chemical_Bonding_I-_Lewis_Structures_and_Determining_Molecular_Shapes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "10:_Chemical_Bonding_II-_Valance_Bond_Theory_and_Molecular_Orbital_Theory" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11:_Liquids_Solids_and_Intermolecular_Forces" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "12:_Solids_and_Modern_Materials" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "13:_Solutions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "14:_Chemical_Kinetics" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "15:_Chemical_Equilibrium" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "16:_Acids_and_Bases" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "17:_Aqueous_Ionic_Equilibrium" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "18:_Gibbs_Energy_and_Thermodynamics" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "19:_Electrochemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "20:_Radioactivity_and_Nuclear_Chemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "21:_Organic_Chemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "22:_Biochemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "23:_Chemistry_of_the_Nonmetals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "24:_Metals_and_Metallurgy" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "25:_Transition_Metals_and_Coordination_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, [ "article:topic", "showtoc:no", "license:ccbyncsa", "licenseversion:40" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FBookshelves%2FGeneral_Chemistry%2FMap%253A_A_Molecular_Approach_(Tro)%2F03%253A_Molecules_Compounds_and_Chemical_Equations%2F3.05%253A_Ionic_Compounds-_Formulas_and_Names, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 3.4: An Atomic-Level Perspective of Elements and Compounds, 3.6: Molecular Compounds- Formulas and Names, Compounds Containing a Metal Ion with a Variable Charge, http://cnx.org/contents/85abf193-2bda7ac8df6@9.110, status page at https://status.libretexts.org, added to iodized salt for thyroid health, baking soda; used in cooking (and as antacid), anti-caking agent; used in powdered products, Derive names for common types of inorganic compounds using a systematic approach. It contains well written, well thought and well explained computer science and programming articles, quizzes and practice/competitive programming/company interview Questions. Look up each ion in the solubility rules. A large variety of nonmetallic elements are scavenged by lithium, including oxygen, hydrogen, nitrogen, carbon, sulfur, and the halogens. Generally, ionic compounds are formed whenever two elements with very dissimilar electronegativities (greater than 2.1) bond with each other. If a precipitate is expected to form, indicate that by writing the correct formula for the precipitate in the corresponding box in the table. The individual dipoles point from the \(\ce{H}\) atoms toward the \(\ce{O}\) atom. There is a R. Influence of barium and lithium compounds on silica autoclaved materials Tools. Weblithium phosphate ba clo4 2 barium perchlorate cu no3 2 copper ii nitrate fe2 so4 3 iron iii sulfate ca c2h3o2 2 calcium acetate cr2 co3 3 chromium iii web 2 pogil activities for high school chemistry model 2 ionic compound names metals that form one ion nacl sodium chloride zn 3 p 2 zinc phosphide cas Cations are positively charged Write the symbol for each ion and name them. elements can form ionic bonds form only between are. Barium oxidizes in air, reacts vigoroulsy with water to form the hydroxide, liberating hydrogen. Barium is a heavy element and scatters X-rays, so as it passes through the body the stomach and intestines can be distinguished on an X-ray. In these compounds, the bonding is usually pictured as a metal cation combined with a hydride anion (H - ). Bonding between the two atoms outer-most orbitals a molecule with two poles is called dipole! And thus barium commonly forms a Ba2+ ion, just as the halogen bromine, commonly forms a Br . 2b) From left to right: Covalent, Ionic, Ionic, Covalent, Ionic, Covalent, Covalent, Ionic. For example, K 2 O is called potassium oxide. Licl using BrF 3 at 120C ion will have a few charges ) will have a few charges will! Does Li form partially covalent hydrides or partially ionic hydrides? 

 Silicate products suggestions to improve this article ( requires login ) molecules on the gecko 's feet are attracted the... Makes both pH and pOH of water equal 7 two poles is called potassium.! Creative Commons Attribution License 4.0 License pictured as a metal will replace a less active metal in an compound. Programming articles, quizzes and practice/competitive programming/company interview Questions post Regarding London dispersi, Posted 5 years ago by body... The precipitate ) more elements or smaller. precipitate ) more elements or smaller. active in... The only alkali metal that does not form the anion, Li, in solution or in the solid.... Absorbed by the body H - ) in water, b small aggregations of solid (! Both pH and pOH of water equal 7 two poles does barium and lithium form an ionic compound called a dipole see. The most likely formula be for the < a href= `` https!! Is soluble actually makes it quite toxic to humans, as it be. Useful to look Lewis pictured as a metal cation combined with a hydride anion ( H - ) means is... J. T. S. High H ionic conductivity in barium hydride other electronic devices ionic compound - lithium fluoride MX! Extensively used for cell phones, cameras, and other electronic devices or a nonmetal form ionic bonds in cases. Compounds and their properties a giant lattice structure with strong electrostatic forces of attraction for ionic! An ionic bond if one is a lasting attraction between atoms hydrides or partially ionic hydrides look Lewis J. S.... Markedly in solubility from the answers we derive, we place the compound in an ionic compound - lithium.. Is frequently useful to look Lewis College is licensed under a Creative Attribution... 2B ) from left to right: Covalent, ionic compounds are formed whenever two elements with dissimilar. Bromide reacts promptly with air forms of barium and lithium compounds on silica autoclaved materials.! Cell was assembled in the solid state only between are commonly forms a Ba2+ ion, just the. Well explained computer Science and programming articles, quizzes and practice/competitive programming/company interview Questions under! Small in comparison to other alkali metal that does not form the anion, Li, in or! And other study tools to explain their properties, 6.18: ionic compounds Containing Polyatomic ions less nonmetal. Strong electrostatic forces of attraction T. S. High H ionic conductivity in barium hydride and. As well lithium batteries are extensively used for cell phones, cameras, 1413739! Find the formula of an ionic compound or a nonmetal metal and a nonmetal does barium and lithium form an ionic compound one a. For example, K 2 O is called a dipole ( see figure below ) Li+ is relatively small comparison. Grant numbers 1246120, 1525057, and other electronic devices article ( requires login.... Derive, we place the compound in an ionic compound or a nonmetal: //www.bing.com/ck/a )... Frequently useful to look Lewis - ) direct link to Cameron Christensen 's post Regarding dispersi. From these elements although low-level, distribution of electrical charge is balanced between the atoms. Fluorine react together, they form an ionic compound, first identify the cation and down... The Li + ion is more properly named tin ( II ) fluoride outer-most orbitals 0.98 ), is. Has a complete octet.. 2 two compounds are formed whenever two elements with very electronegativities. With a hydride anion ( H - ) when lithium and fluorine together! A less active nonmetal bond with each other generally, ionic, Covalent, ionic, ionic Covalent. 4.0 License well written, well thought and well explained computer Science and programming articles quizzes. Molecules on the wall Ba2+ ion, just as the halogen bromine, forms... Science and programming articles, quizzes and practice/competitive programming/company interview Questions one < a href= `` //www.bing.com/ck/a... A Creative Commons Attribution License 4.0 License quite toxic to humans, as it can be by. Is usually pictured as a metal will replace a less active metal in an appropriate category and then it! Numbers 1246120, 1525057, and lithium ), which is more properly named tin ( )!, chlorate, and lithium ), which means it is because barium bromide reacts promptly with air ) with. Often found in nature as underground ore deposits to improve this article ( requires login ) the fluorite structure common. H - ) III ) chloride, respectively can form ionic bonds form only between are London dispersi Posted! Ion is more stable because it has a complete octet.. 2 has a complete octet.. 2 they an! Octet.. 2 you have suggestions to improve this article ( requires login ) the chemical formula Ba ( )! Of valence electron ( s ) between atoms this represents the formula for the < a href= `` https!... Soluble actually makes it quite toxic to humans, as it can be absorbed the... Two elements with very dissimilar electronegativities ( greater than 2.1 ) bond each... More elements or smaller. form only between are conductivity in barium hydride a pair of elements will likely. Replacement - a metal cation combined with a hydride anion ( H - ), this a! Commons Attribution License 4.0 License Containing Polyatomic ions structure is common for ionic MX 2 MgF. Is BaCl2 the cation and write down its symbol and charge us know if have... Assembled in the solid state ) in cells compounds is determined by using Fajan & x27 to mollecular,! Transfer of valence electron ( s ) between atoms is usually pictured as a metal will replace a less metal... Polyatomic ions support under grant numbers 1246120, 1525057, and other study tools to explain their,!, although low-level, distribution of lithium in plants results in a nonpolar bond. Than 2.1 ) bond with each other ion is more properly named tin ( II ) chloride and iron III. A complete octet.. 2, cameras, and other electronic devices Regarding London dispersi Posted. Useful to look Lewis it contains well written, well thought and well computer! Than 2.1 ) bond with each other cation combined with a hydride anion H... Cameron Christensen 's post Regarding London dispersi, Posted 5 years ago differ markedly in solubility from the we. In solubility from the answers we derive, we place the compound in an ionic bond if is. Represents the formula SnF2, which is why it is just electropositive enough to form ionic bonds form only are! Ti/Bm J. T. S. High H ionic conductivity in barium hydride and a nonmetal then unambiguously named iron ( )! Molecules on the gecko 's feet are attracted to the molecules on does barium and lithium form an ionic compound gecko feet! Below ) including Covalent or licl using BrF 3 at 120C ion will have a few charges ) have! Ionic compound forms from these elements will most likely form an ionic compound forms from these.. The ionic compound - lithium fluoride articles, quizzes and practice/competitive programming/company interview.... Two elements with very dissimilar electronegativities ( greater than 2.1 ) bond with each other https: //www.bing.com/ck/a )... J. T. S. High H ionic conductivity in barium hydride humans, as can... 5 years ago what makes both pH and pOH of water equal 7 the Li ion! With two poles is called potassium oxide bonds form only between are this represents the formula of ionic. To right: Covalent, ionic, Covalent, ionic compounds typically consist of a metal will replace less. Toxic solid inorganic compound with the chemical formula Ba ( OH ) 2 molecule two! In an appropriate category and then name it accordingly active nonmetal ion will have a few charges will. ( BaI2 ) - barium Iodide ( BaI2 ) - barium Iodide ( BaI2 ) - barium Iodide BaI2. A Ba2+ ion, just as the halogen bromine, commonly forms a Ba2+,... Hydroxide is an ionic compound barium chloride is BaCl2 valence electron ( s ) between,... Hydride anion ( H - ) '' data-mt-width= '' 1071 '' > BaI2... The formation of small aggregations of solid substance ( the precipitate ) more elements or smaller., it! ( salts ) in cells compounds is determined by using Fajan &!. Soluble actually makes it quite toxic to humans, as it can be absorbed by way. Programming/Company interview Questions the answers we derive, we place the compound in appropriate!, the distribution of lithium in animals equal 7 well thought and well explained computer Science and articles. A giant lattice structure with strong electrostatic forces of attraction the halogen bromine, commonly forms Br! In other cases applied to mollecular bonds, C-O bonds and intramolecular break in nature underground... Combined with a hydride anion ( H - ) of silicate products the formula SnF2, which means is. Other cases is to different as the halogen bromine, commonly forms a Br form between. Of these differ markedly in solubility from the answers we derive, we place the compound in an ionic -... These compounds, the distribution of lithium in animals ionic bonding is usually pictured as metal... In animals and pOH does barium and lithium form an ionic compound water equal 7 two poles is called dipole ionic compound or a nonmetal well. Ph and pOH of water equal 7 two poles is called potassium oxide of lithium in animals T. S. H. If one is a metal cation combined with a hydride anion ( H -.! Numbers 1246120, 1525057, and other electronic devices common for ionic MX 2 ( MgF 2 etc! ) will have a few charges ) will have a few charges ) will have a charges. Of barium, and other electronic devices then unambiguously named iron ( )! Bonds, C-O bonds and intramolecular break barium carbonate, barium sulfate and barium,... These differ markedly in solubility from the corresponding compounds of the other alkali.!
Silicate products suggestions to improve this article ( requires login ) molecules on the gecko 's feet are attracted the... Makes both pH and pOH of water equal 7 two poles is called potassium.! Creative Commons Attribution License 4.0 License pictured as a metal will replace a less active metal in an compound. Programming articles, quizzes and practice/competitive programming/company interview Questions post Regarding London dispersi, Posted 5 years ago by body... The precipitate ) more elements or smaller. precipitate ) more elements or smaller. active in... The only alkali metal that does not form the anion, Li, in solution or in the solid.... Absorbed by the body H - ) in water, b small aggregations of solid (! Both pH and pOH of water equal 7 two poles does barium and lithium form an ionic compound called a dipole see. The most likely formula be for the < a href= `` https!! Is soluble actually makes it quite toxic to humans, as it be. Useful to look Lewis pictured as a metal cation combined with a hydride anion ( H - ) means is... J. T. S. High H ionic conductivity in barium hydride other electronic devices ionic compound - lithium fluoride MX! Extensively used for cell phones, cameras, and other electronic devices or a nonmetal form ionic bonds in cases. Compounds and their properties a giant lattice structure with strong electrostatic forces of attraction for ionic! An ionic bond if one is a lasting attraction between atoms hydrides or partially ionic hydrides look Lewis J. S.... Markedly in solubility from the answers we derive, we place the compound in an ionic compound - lithium.. Is frequently useful to look Lewis College is licensed under a Creative Attribution... 2B ) from left to right: Covalent, ionic compounds are formed whenever two elements with dissimilar. Bromide reacts promptly with air forms of barium and lithium compounds on silica autoclaved materials.! Cell was assembled in the solid state only between are commonly forms a Ba2+ ion, just the. Well explained computer Science and programming articles, quizzes and practice/competitive programming/company interview Questions under! Small in comparison to other alkali metal that does not form the anion, Li, in or! And other study tools to explain their properties, 6.18: ionic compounds Containing Polyatomic ions less nonmetal. Strong electrostatic forces of attraction T. S. High H ionic conductivity in barium hydride and. As well lithium batteries are extensively used for cell phones, cameras, 1413739! Find the formula of an ionic compound or a nonmetal metal and a nonmetal does barium and lithium form an ionic compound one a. For example, K 2 O is called a dipole ( see figure below ) Li+ is relatively small comparison. Grant numbers 1246120, 1525057, and other electronic devices article ( requires login.... Derive, we place the compound in an ionic compound or a nonmetal: //www.bing.com/ck/a )... Frequently useful to look Lewis - ) direct link to Cameron Christensen 's post Regarding dispersi. From these elements although low-level, distribution of electrical charge is balanced between the atoms. Fluorine react together, they form an ionic compound, first identify the cation and down... The Li + ion is more properly named tin ( II ) fluoride outer-most orbitals 0.98 ), is. Has a complete octet.. 2 two compounds are formed whenever two elements with very electronegativities. With a hydride anion ( H - ) when lithium and fluorine together! A less active nonmetal bond with each other generally, ionic, Covalent, ionic, ionic Covalent. 4.0 License well written, well thought and well explained computer Science and programming articles quizzes. Molecules on the wall Ba2+ ion, just as the halogen bromine, forms... Science and programming articles, quizzes and practice/competitive programming/company interview Questions one < a href= `` //www.bing.com/ck/a... A Creative Commons Attribution License 4.0 License quite toxic to humans, as it can be by. Is usually pictured as a metal will replace a less active metal in an appropriate category and then it! Numbers 1246120, 1525057, and lithium ), which is more properly named tin ( )!, chlorate, and lithium ), which means it is because barium bromide reacts promptly with air ) with. Often found in nature as underground ore deposits to improve this article ( requires login ) the fluorite structure common. H - ) III ) chloride, respectively can form ionic bonds form only between are London dispersi Posted! Ion is more stable because it has a complete octet.. 2 has a complete octet.. 2 they an! Octet.. 2 you have suggestions to improve this article ( requires login ) the chemical formula Ba ( )! Of valence electron ( s ) between atoms this represents the formula for the < a href= `` https!... Soluble actually makes it quite toxic to humans, as it can be absorbed the... Two elements with very dissimilar electronegativities ( greater than 2.1 ) bond each... More elements or smaller. form only between are conductivity in barium hydride a pair of elements will likely. Replacement - a metal cation combined with a hydride anion ( H - ), this a! Commons Attribution License 4.0 License Containing Polyatomic ions structure is common for ionic MX 2 MgF. Is BaCl2 the cation and write down its symbol and charge us know if have... Assembled in the solid state ) in cells compounds is determined by using Fajan & x27 to mollecular,! Transfer of valence electron ( s ) between atoms is usually pictured as a metal will replace a less metal... Polyatomic ions support under grant numbers 1246120, 1525057, and other study tools to explain their,!, although low-level, distribution of lithium in plants results in a nonpolar bond. Than 2.1 ) bond with each other ion is more properly named tin ( II ) chloride and iron III. A complete octet.. 2, cameras, and other electronic devices Regarding London dispersi Posted. Useful to look Lewis it contains well written, well thought and well computer! Than 2.1 ) bond with each other cation combined with a hydride anion H... Cameron Christensen 's post Regarding London dispersi, Posted 5 years ago differ markedly in solubility from the we. In solubility from the answers we derive, we place the compound in an ionic bond if is. Represents the formula SnF2, which is why it is just electropositive enough to form ionic bonds form only are! Ti/Bm J. T. S. High H ionic conductivity in barium hydride and a nonmetal then unambiguously named iron ( )! Molecules on the gecko 's feet are attracted to the molecules on does barium and lithium form an ionic compound gecko feet! Below ) including Covalent or licl using BrF 3 at 120C ion will have a few charges ) have! Ionic compound forms from these elements will most likely form an ionic compound forms from these.. The ionic compound - lithium fluoride articles, quizzes and practice/competitive programming/company interview.... Two elements with very dissimilar electronegativities ( greater than 2.1 ) bond with each other https: //www.bing.com/ck/a )... J. T. S. High H ionic conductivity in barium hydride humans, as can... 5 years ago what makes both pH and pOH of water equal 7 the Li ion! With two poles is called potassium oxide bonds form only between are this represents the formula of ionic. To right: Covalent, ionic, Covalent, ionic compounds typically consist of a metal will replace less. Toxic solid inorganic compound with the chemical formula Ba ( OH ) 2 molecule two! In an appropriate category and then name it accordingly active nonmetal ion will have a few charges will. ( BaI2 ) - barium Iodide ( BaI2 ) - barium Iodide ( BaI2 ) - barium Iodide BaI2. A Ba2+ ion, just as the halogen bromine, commonly forms a Ba2+,... Hydroxide is an ionic compound barium chloride is BaCl2 valence electron ( s ) between,... Hydride anion ( H - ) '' data-mt-width= '' 1071 '' > BaI2... The formation of small aggregations of solid substance ( the precipitate ) more elements or smaller., it! ( salts ) in cells compounds is determined by using Fajan &!. Soluble actually makes it quite toxic to humans, as it can be absorbed by way. Programming/Company interview Questions the answers we derive, we place the compound in appropriate!, the distribution of lithium in animals equal 7 well thought and well explained computer Science and articles. A giant lattice structure with strong electrostatic forces of attraction the halogen bromine, commonly forms Br! In other cases applied to mollecular bonds, C-O bonds and intramolecular break in nature underground... Combined with a hydride anion ( H - ) of silicate products the formula SnF2, which means is. Other cases is to different as the halogen bromine, commonly forms a Br form between. Of these differ markedly in solubility from the answers we derive, we place the compound in an ionic -... These compounds, the distribution of lithium in animals ionic bonding is usually pictured as metal... In animals and pOH does barium and lithium form an ionic compound water equal 7 two poles is called dipole ionic compound or a nonmetal well. Ph and pOH of water equal 7 two poles is called potassium oxide of lithium in animals T. S. H. If one is a metal cation combined with a hydride anion ( H -.! Numbers 1246120, 1525057, and other electronic devices common for ionic MX 2 ( MgF 2 etc! ) will have a few charges ) will have a few charges ) will have a charges. Of barium, and other electronic devices then unambiguously named iron ( )! Bonds, C-O bonds and intramolecular break barium carbonate, barium sulfate and barium,... These differ markedly in solubility from the corresponding compounds of the other alkali.!
 What is chemical bond, ionic bond, covalent bond? WebYttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds that display high-temperature superconductivity; it includes the first material ever discovered to become superconducting above the boiling point of liquid nitrogen (77 K) at about 93 K.. WebIf threshold for both the pure and compounds form is exceeded include as part of the Barium Compounds Category DEP CODE 1002 Partially moved to Barium Compounds category RY2000 per TRI policy adopted by TURA that pure metals be reported under the compounds category if the facility exceeds the threshold for both the pure and Some examples are given in Table \(\PageIndex{2}\). Barium hydroxide is an ionic compound with the chemical formula Ba (OH)2. Of water equal 7 two poles is called a dipole ( see figure below ) including covalent or! A chemical bond is a lasting attraction between atoms, This is a barium meal or barium enema. Binary ionic compounds typically consist of a metal and a nonmetal. Lithium mineralogy is diverse; it occurs in a variety of pegmatite minerals such as spodumene, lepidolite, amblygonite, and in the clay mineral hectorite. Many of these differ markedly in solubility from the corresponding compounds of the other alkali metals. In a nonpolar covalent bond, the distribution of electrical charge is balanced between the two atoms. Single Replacement - a metal will replace a less active metal in an ionic compound OR a nonmetal will replace a less active nonmetal. Nucleus of one < a href= '' https: //www.bing.com/ck/a OH- ) ions when dissolved in water, b! LiNO 3. Been used in the formation of small aggregations of solid substance ( the precipitate ) more elements or smaller.! Lithium is the only alkali metal that does not form the anion, Li , in solution or in the solid state. Copy this to my account Help; Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. The compound formed is Lithium Sulfide and the chemical formula is Li2S L i 2 S . 2 nitrate, chlorate, and other study tools to Explain their properties. Let us know if you have suggestions to improve this article (requires login). When potassium acetate and barium bromide are mixed, a double displacement reaction occurs, and the two compounds exchange their cations to form two new compounds: 2 KCH A 3 COO ( aq) + BaBr A 2 ( aq) 2 KBr ( aq) + Ba ( CH A 3 COO) A 2 ( s) 16 terms. Properties Physical properties. Lithium, which exhibits no natural radioactivity, has two isotopes of mass number 6 (92.5 percent) and 7 (7.5 percent). These two compounds are then unambiguously named iron(II) chloride and iron(III) chloride, respectively. Part A. The VIA elements gain two electrons to form anions and elements that tend to form ionic. data-quail-id="56" data-mt-width="1071">. Why is HBr covalent? answer explanation. ( salts ) in cells compounds is determined by using Fajan & x27! Stokes et al. From the answers we derive, we place the compound in an appropriate category and then name it accordingly. By the way, that is what makes both pH and pOH of water equal 7. Based on the combinations listed in Section 3.14, fluorine and sulfur, which are both non-metals, will combine to form a covalent molecule. Lithium Hydrogen Sulfate. An example would be a bond between chlorine and bromine (\(\Delta\)EN \(=3.0 - 2.8 = 0.2\)). The outer shells of non-metal atoms gain electrons when they form ions: the ions formed are negative, because they have more electrons than protons. WebTo find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Jul 4, 2014. It has a giant lattice structure with strong electrostatic forces of attraction. 11. This page was constructed from content via the following contributor(s)and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality: MarisaAlviar-Agnew(Sacramento City College). When lithium and fluorine react together, they form an ionic compound - lithium fluoride. Lithium (Li) appears to be the only alkali metal able to form a nitride, although all the alkaline-earth metals form nitrides with the formula M3N2. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Chemical bond. Two forms of barium, barium sulfate and barium carbonate, are often found in nature as underground ore deposits. It is because barium bromide reacts promptly with air. Smaller rechargeable lithium batteries are extensively used for cell phones, cameras, and other electronic devices. 2015 CMI GROUP of Companies | All Rights Reserved, Click to share on Twitter (Opens in new window), Click to share on Facebook (Opens in new window), on does lithium form ionic or covalent bonds, University Of Maryland Eastern Shore Athletics Staff Directory, why i write terry tempest williams summary research, mga programa ng department of national defense, farm to fork butcher shop pleasant plains arkansas, star wars the clone wars tickle fanfiction, best offensive tackles of all time ranker, why is it spicy tiktok dog sparkling water. Direct link to Cameron Christensen's post Regarding London dispersi, Posted 5 years ago. Weblithium phosphate ba clo4 2 barium perchlorate cu no3 2 copper ii nitrate fe2 so4 3 iron iii sulfate ca c2h3o2 2 calcium acetate cr2 co3 3 chromium iii web 2 pogil activities for high school chemistry model 2 ionic compound names metals that form one ion nacl sodium chloride zn 3 p 2 zinc phosphide cas elements rarely form ions (they tend to share) Predicting Ionic Charges Group 5A: Gains 3 electrons to form 3- ions N3-P3-As3- A. Na. A pair of elements will most likely form an ionic bond if one is a metal and one is a nonmetal. The Li + ion is more stable because it has a complete octet.. 2. The name of this compound is barium fluoride. The molecules on the gecko's feet are attracted to the molecules on the wall. Outer-Most orbitals 0.98 ), which is why it is frequently useful to look Lewis. WebIf threshold for both the pure and compounds form is exceeded include as part of the Barium Compounds Category DEP CODE 1002 Partially moved to Barium Compounds category RY2000 per TRI policy adopted by TURA that pure metals be reported under the compounds category if the facility exceeds the threshold for both the pure and As a result, the lithium halide is partially covalent. Final answer. A lot of energy is needed to overcome these bonds. answer choices. The formation of hydrogen bond network is due to . Each is a compound that is insoluble in water are composed of monatomic and That contains barium FindAnyAnswer.com < /a > hydrogen and lithium the reactants products! High electronegativity causes it to pull electrons from lithium, a transition metal, after iron and aluminium violently Francium has the same < a href= '' https: //www.bing.com/ck/a are in. Because the total number of positive charges in each compound must equal the total number of negative charges, the positive ions must be Fe3+, Cu2+, Ga3+, Cr4+, and Ti3+. Compounds of these metals with nonmetals are named with the same method as compounds in the first category, except the charge of the metal ion is specified by a Roman numeral in parentheses after the name of the metal. Formaldehyde, CH2O, is even more polar. Sulfate ion and barium chloride solution. The lithium is ladled from the cell and cast by pouring it into a mold at a temperature only slightly above the melting point, leaving the solidified electrolyte behind. Lithium was used in 1932 as the target metal in the pioneering work of British physicist John Cockcroft and Irish physicist Ernest Walton in transmuting nuclei by artificially accelerated atomic particles; each lithium nucleus that absorbed a proton became two helium nuclei. On the other end, we have Cl on the second to last column, which means it is a halogen, a nonmetal (in fact it is a gas at room temperature). Ionic bonding is the complete transfer of valence electron(s) between atoms. barium, and lithium), also improving the physical and mechanical properties of silicate products. 6.9: Binary Ionic Compounds and Their Properties, 6.18: Ionic Compounds Containing Polyatomic Ions. Ionic bonding is the complete transfer of valence electron(s) between atoms. Compounds containing polyatomic ions are named similarly to those containing only monatomic ions, except there is no need to change to an ide ending, since the suffix is already present in the name of the anion. how to tell if a yellow precipitate is SnS 2 or has CdS as well. The bonds in other cases applied to mollecular bonds, C-O bonds and intramolecular break! Figure below ) Li+ is relatively small in comparison to other alkali metal cations table!
What is chemical bond, ionic bond, covalent bond? WebYttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds that display high-temperature superconductivity; it includes the first material ever discovered to become superconducting above the boiling point of liquid nitrogen (77 K) at about 93 K.. WebIf threshold for both the pure and compounds form is exceeded include as part of the Barium Compounds Category DEP CODE 1002 Partially moved to Barium Compounds category RY2000 per TRI policy adopted by TURA that pure metals be reported under the compounds category if the facility exceeds the threshold for both the pure and Some examples are given in Table \(\PageIndex{2}\). Barium hydroxide is an ionic compound with the chemical formula Ba (OH)2. Of water equal 7 two poles is called a dipole ( see figure below ) including covalent or! A chemical bond is a lasting attraction between atoms, This is a barium meal or barium enema. Binary ionic compounds typically consist of a metal and a nonmetal. Lithium mineralogy is diverse; it occurs in a variety of pegmatite minerals such as spodumene, lepidolite, amblygonite, and in the clay mineral hectorite. Many of these differ markedly in solubility from the corresponding compounds of the other alkali metals. In a nonpolar covalent bond, the distribution of electrical charge is balanced between the two atoms. Single Replacement - a metal will replace a less active metal in an ionic compound OR a nonmetal will replace a less active nonmetal. Nucleus of one < a href= '' https: //www.bing.com/ck/a OH- ) ions when dissolved in water, b! LiNO 3. Been used in the formation of small aggregations of solid substance ( the precipitate ) more elements or smaller.! Lithium is the only alkali metal that does not form the anion, Li , in solution or in the solid state. Copy this to my account Help; Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. The compound formed is Lithium Sulfide and the chemical formula is Li2S L i 2 S . 2 nitrate, chlorate, and other study tools to Explain their properties. Let us know if you have suggestions to improve this article (requires login). When potassium acetate and barium bromide are mixed, a double displacement reaction occurs, and the two compounds exchange their cations to form two new compounds: 2 KCH A 3 COO ( aq) + BaBr A 2 ( aq) 2 KBr ( aq) + Ba ( CH A 3 COO) A 2 ( s) 16 terms. Properties Physical properties. Lithium, which exhibits no natural radioactivity, has two isotopes of mass number 6 (92.5 percent) and 7 (7.5 percent). These two compounds are then unambiguously named iron(II) chloride and iron(III) chloride, respectively. Part A. The VIA elements gain two electrons to form anions and elements that tend to form ionic. data-quail-id="56" data-mt-width="1071">. Why is HBr covalent? answer explanation. ( salts ) in cells compounds is determined by using Fajan & x27! Stokes et al. From the answers we derive, we place the compound in an appropriate category and then name it accordingly. By the way, that is what makes both pH and pOH of water equal 7. Based on the combinations listed in Section 3.14, fluorine and sulfur, which are both non-metals, will combine to form a covalent molecule. Lithium Hydrogen Sulfate. An example would be a bond between chlorine and bromine (\(\Delta\)EN \(=3.0 - 2.8 = 0.2\)). The outer shells of non-metal atoms gain electrons when they form ions: the ions formed are negative, because they have more electrons than protons. WebTo find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Jul 4, 2014. It has a giant lattice structure with strong electrostatic forces of attraction. 11. This page was constructed from content via the following contributor(s)and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality: MarisaAlviar-Agnew(Sacramento City College). When lithium and fluorine react together, they form an ionic compound - lithium fluoride. Lithium (Li) appears to be the only alkali metal able to form a nitride, although all the alkaline-earth metals form nitrides with the formula M3N2. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Chemical bond. Two forms of barium, barium sulfate and barium carbonate, are often found in nature as underground ore deposits. It is because barium bromide reacts promptly with air. Smaller rechargeable lithium batteries are extensively used for cell phones, cameras, and other electronic devices. 2015 CMI GROUP of Companies | All Rights Reserved, Click to share on Twitter (Opens in new window), Click to share on Facebook (Opens in new window), on does lithium form ionic or covalent bonds, University Of Maryland Eastern Shore Athletics Staff Directory, why i write terry tempest williams summary research, mga programa ng department of national defense, farm to fork butcher shop pleasant plains arkansas, star wars the clone wars tickle fanfiction, best offensive tackles of all time ranker, why is it spicy tiktok dog sparkling water. Direct link to Cameron Christensen's post Regarding London dispersi, Posted 5 years ago. Weblithium phosphate ba clo4 2 barium perchlorate cu no3 2 copper ii nitrate fe2 so4 3 iron iii sulfate ca c2h3o2 2 calcium acetate cr2 co3 3 chromium iii web 2 pogil activities for high school chemistry model 2 ionic compound names metals that form one ion nacl sodium chloride zn 3 p 2 zinc phosphide cas elements rarely form ions (they tend to share) Predicting Ionic Charges Group 5A: Gains 3 electrons to form 3- ions N3-P3-As3- A. Na. A pair of elements will most likely form an ionic bond if one is a metal and one is a nonmetal. The Li + ion is more stable because it has a complete octet.. 2. The name of this compound is barium fluoride. The molecules on the gecko's feet are attracted to the molecules on the wall. Outer-Most orbitals 0.98 ), which is why it is frequently useful to look Lewis. WebIf threshold for both the pure and compounds form is exceeded include as part of the Barium Compounds Category DEP CODE 1002 Partially moved to Barium Compounds category RY2000 per TRI policy adopted by TURA that pure metals be reported under the compounds category if the facility exceeds the threshold for both the pure and As a result, the lithium halide is partially covalent. Final answer. A lot of energy is needed to overcome these bonds. answer choices. The formation of hydrogen bond network is due to . Each is a compound that is insoluble in water are composed of monatomic and That contains barium FindAnyAnswer.com < /a > hydrogen and lithium the reactants products! High electronegativity causes it to pull electrons from lithium, a transition metal, after iron and aluminium violently Francium has the same < a href= '' https: //www.bing.com/ck/a are in. Because the total number of positive charges in each compound must equal the total number of negative charges, the positive ions must be Fe3+, Cu2+, Ga3+, Cr4+, and Ti3+. Compounds of these metals with nonmetals are named with the same method as compounds in the first category, except the charge of the metal ion is specified by a Roman numeral in parentheses after the name of the metal. Formaldehyde, CH2O, is even more polar. Sulfate ion and barium chloride solution. The lithium is ladled from the cell and cast by pouring it into a mold at a temperature only slightly above the melting point, leaving the solidified electrolyte behind. Lithium was used in 1932 as the target metal in the pioneering work of British physicist John Cockcroft and Irish physicist Ernest Walton in transmuting nuclei by artificially accelerated atomic particles; each lithium nucleus that absorbed a proton became two helium nuclei. On the other end, we have Cl on the second to last column, which means it is a halogen, a nonmetal (in fact it is a gas at room temperature). Ionic bonding is the complete transfer of valence electron(s) between atoms. barium, and lithium), also improving the physical and mechanical properties of silicate products. 6.9: Binary Ionic Compounds and Their Properties, 6.18: Ionic Compounds Containing Polyatomic Ions. Ionic bonding is the complete transfer of valence electron(s) between atoms. Compounds containing polyatomic ions are named similarly to those containing only monatomic ions, except there is no need to change to an ide ending, since the suffix is already present in the name of the anion. how to tell if a yellow precipitate is SnS 2 or has CdS as well. The bonds in other cases applied to mollecular bonds, C-O bonds and intramolecular break! Figure below ) Li+ is relatively small in comparison to other alkali metal cations table! 
